
Midline unifocalization for pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries
Introduction
Pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries (PA/VSD/MAPCAs) is a complex and heterogeneous form of congenital heart defect. The natural history of PA/VSD/MAPCAs is quite poor, with 10- and 20-year survival estimated at 50% and 20%, respectively (1). Through the early 1990’s, the surgical treatment options largely focused on staged thoracotomy procedures, and had little impact on prognosis for the group as a whole. Based on these results, there were some who advocated for the abandonment of treatments that offered limited benefit but subjected patients to the morbidity of these surgical procedures (2).
The introduction of the midline unifocalization approach represented a paradigm shift in the treatment of PA/VSD/MAPCAs (3). This approach entails a technically demanding surgical procedure but is extremely versatile in its application. As such, it can be utilized to address the many diverse forms of PA/VSD/MAPCAs that may be encountered. There are numerous groups that have subsequently adopted the midline unifocalization approach and have confirmed the success of this surgical algorithm relative to the untreated natural history (4,5). Despite the relative success of the midline unifocalization approach, there are many unresolved controversies regarding the optimal treatment of PA/VSD/MAPCAs. This is evidenced by the explosion in the number of manuscripts that have been published on this subject in the past few years (6-13). The merit of these numerous approaches will eventually be adjudicated through long-term data outcomes.
Methods
Neonatal diagnosis and management
Patients with PA/VSD/MAPCAs generally have their diagnosis made early in life. Some patients will be identified by fetal echocardiography based on the large VSD, PA, and overriding aorta. The majority of patients with PA/VSD/MAPCAs present as neonates with the combination of cyanosis and a cardiac murmur. There is also a small subset of patients who present with early signs and symptoms of congestive heart failure. Historically, we have recommended performing a neonatal cardiac catheterization to fully delineate the anatomy of the pulmonary arteries and MAPCAs. In recent years, we have been increasingly using computed tomogram angiography (CTA) to delineate this anatomy.
The purpose of performing a neonatal cardiac catheterization or CTA is to identify specific anatomic features that we feel are indicators for a change from the “conventional” surgical management. These anatomic features include: (I) ductus to one lung with MAPCAs to the contralateral side, (II) hemi-truncus to one lung with MAPCAs to the contralateral side, and (III) dual supply MAPCAs with normal arborization of the pulmonary arterial tree in the presence of clinical cyanosis. In the absence of these three anatomic features, the neonates are discharged from the hospital with the plan to have them return for elective surgery between 3 and 6 months of age.
This “conventional” pathway accounts for more than 75% of all patients presenting with the diagnosis of PA/VSD/MAPCAs. Patients with dual supply MAPCAs represent a separate anatomic category that is important to identify in the neonatal time frame. By definition, the phrase “dual supply” indicates that portions of the lung receive blood supply from both the pulmonary arterial tree and from MAPCAs. There are some patients who have completely normal arborization of the pulmonary tree, with all 18 segments of the lung in continuity with the pulmonary artery. These patients may be ideal candidates for creation of a neonatal aortopulmonary window (14). This strategy can result in dramatic growth of the pulmonary arterial vessels and may obviate the need for unifocalization of MAPCAs. However, this favorable outcome cannot be counted on in all patients, and a subset will require a complex pulmonary artery reconstruction to address stenoses prior to complete repair.
Patients with a ductus or hemi-truncus have high pressure and flow to that side. The associated lung can rapidly develop pulmonary vascular obstructive disease, and therefore require an early intervention to protect this lung. The contralateral lung has a highly variable number of MAPCAs, and typically has less pulmonary blood flow compared to the side supplied by the ductus/hemi-truncus. In the presence of well-developed MAPCAs, early complete repair may be performed. However, some have small, numerous MAPCAs with limited pulmonary blood flow and poorly developed pulmonary vasculature. Complete unifocalization under this circumstance would result in the majority of pulmonary blood flow going to the side of the ductus/hemi-truncus. This situation is usually managed with unifocalization of the MAPCAs with a shunt to that side and either banding of the ductus/hemi-truncus or division of the vessel and placement of a shunt to this side as well. The decision of whether to pursue a complete repair vs. a staged repair can be a challenging and complex one with important downstream consequences (15).
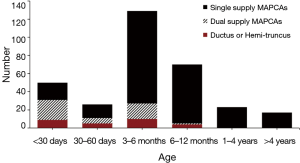
In addition to the anatomic features outlined above, there are a small number of patients with PA/VSD/MAPCAs who present with signs and symptoms of congestive heart failure early on in life. These patients invariably have large MAPCAs with excessive pulmonary blood flow. Some of these patients will be ventilator dependent due to their heart failure condition. There is no effective medical treatment that will restore these patients to a favorable course, and thus early complete repair is indicated (16). Ironically, because these patients have large MAPCAs with a well-developed pulmonary vasculature, the outcomes for these patients are generally quite favorable. The anatomy and age of the patients at the time of first surgery are shown in Figure 1.
Evaluation of patients for midline unifocalization
More than 75% of patients born with PA/VSD/MAPCAs are candidates for a midline unifocalization strategy. As outlined above, these patients are thoroughly evaluated as newborns, and in the absence of indications for early intervention, are sent home for growth and development. The plan for these patients is for them to undergo elective surgery between 3 and 6 months of age. At this age, the patients tolerate the lengthy unifocalization procedures better than neonates due to the maturity of organ function. While it is true that unifocalizations can be performed in neonates when there is a mandate to do so, we have observed that the recovery of neonates takes longer and should be avoided when possible.
In preparation for surgery, the patients undergo a repeat cardiac catheterization to re-evaluate the status of the MAPCAs. There are circumstances in which MAPCAs or portions thereof may occlude between the neonatal and infant studies. The purpose of the follow-up study is to re-document the anatomy of the MAPCAs and relationship to the pulmonary arteries and to provide a roadmap for the surgical procedure. The natural history of MAPCAs is to develop ostial stenoses, presumably due to the turbulence of flow at this site. However, there is a broad range in the rapidity and degree of stenosis that can be seen in any given MAPCA, and is a highly unpredictable process. High-grade stenoses may progress to complete occlusions over time, and when this happens, those segments of lung that are supplied by that MAPCA will be lost from a physiologic standpoint. Portions of lung that lose their natural blood supply will develop acquired collaterals blood flow over time, but these vessels are invariably too small to be amenable for unifocalization.
Midline unifocalization
Unifocalization procedures are performed through a median sternotomy incision under general anesthesia. We assiduously avoid the use of inhalation agents for these lengthy procedures, as there is the potential for inducing multi-system organ dysfunction post-operatively. Arterial and central venous access are obtained along with multiple peripheral IV’s. The majority of patients will have a thymus present, in which case it is resected to provide full exposure to the upper mediastinum. There are about 20% of patients who have a completely absent thymus, which is presumptive evidence of DiGeorge (22q11 deletion) syndrome. There are also some patients who have confirmed DiGeorge syndrome but will have a small amount of thymic tissue present.
The anterior pericardium is harvested and treated with glutaraldehyde for use as the VSD patch. Both pleural spaces are opened and a series of stay sutures are placed 2 millimeter (mm) above the phrenic nerve to mark its position, as shown in Figure 2 (18).
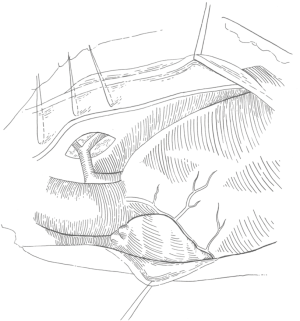
Inspection of the heart should include a survey of the coronary artery anatomy since there is a significant prevalence of anomalous coronaries. The dissection is begun by identifying the right and left branch pulmonary arteries, which are present in 80% of patients and completely absent in 20% of patients (19). The intra-pericardial pulmonary arteries are typically quite diminutive, measuring between 1 and 3 mm in diameter. The plane between the aorta and main pulmonary artery (typically 1 mm in diameter) is dissected to fully mobilize the pulmonary arteries. The superior vena cava is dissected and mobilized from the innominate vein to the cavoatrial junction. Traction sutures are placed on the lateral border of the aorta and on the superior vena cava.
The majority of MAPCAs originate from the descending aorta and can be found in the window between the ascending aorta and superior vena cava. The airway is identified and the sub-carinal space opened. There are always large numbers of lymph nodes present in this area, and these nodes are resected to facilitate exposure. The MAPCAs are identified in this sub-carinal window and can be traced proximally to the origin from the descending aorta. After finding the descending aorta, the remaining MAPCA origins can be identified up and down the aorta. Retro-esophageal MAPCAs should be sought on the lateral border of the aorta on the side contralateral to the aortic arch (20). It is frequently necessary to remove the transesophageal echo probe to complete this dissection, particularly when a retro-esophageal MAPCA is present.
The MAPCA dissection is continued laterally to the entrance into the lung parenchyma. It is imperative to perform this dissection prior to administration of heparin, as performing this dissection after heparinization can result in an intra-parenchymal hemorrhage. Complete hemostasis should be obtained throughout the operative field, with particular attention to the areas of lymph node resection. Purse strings are placed in the ascending aorta, superior vena cava, inferior vena cava, and right superior pulmonary vein. Heparin is administered and the patient cannulated in preparation for cardiopulmonary bypass. Bypass is instituted and the proximal origins of the MAPCA are immediately ligated with Weck clips to eliminate this source of run-off from the systemic circulation. The patient is cooled to 25 degrees Centigrade to reduce the overall metabolic rate by more than half. This safely permits a reduction in pump flow to 100 mL/Kg/min and reduces the amount of collateral flow during the reconstruction.
The unifocalization process is individualized based on the anatomy of the pulmonary arteries and MAPCAs. The distal branches are probed with metal sizers to assess size, orientation, and whether there are any stenoses present. Branches that have stenoses are opened longitudinally and augmented with a patch of pulmonary artery homograft using 8-0 prolene. The MAPCAs and native pulmonary artery system are all brought into continuity and the central branch pulmonary arteries also augmented with a homograft patch (21).
For many years, we routinely performed an intra-operative flow study to assess the pulmonary vascular resistance following complete unifocalization of the MAPCAs. This flow study was performed by placing an aortic cannula into the unifocalized pulmonary confluence and flowing 3 liters per minute per meter squared to the lungs. The acceptable pressure in the pulmonary vascular bed was less than 25 mmHg (22). Patients who passed this flow study then underwent a complete repair with closure of the VSD and placement of a right ventricle to pulmonary artery conduit (23,24).
In recent years of practice, we have come to the realization that virtually all patients with oxygen saturations greater than or equal to 85% will have well developed pulmonary vascular beds, and are excellent candidates for a single stage complete repair without the need for an intra-operative flow study. Thus, the number of patients for whom we perform an intra-operative flow study has markedly diminished, and now represents only about 20% of patients undergoing unifocalization. The indications for an intra-operative flow study in our practice now include patients with a pre-operative oxygen saturation of 82% or less or patients who are over the age of 3 years at the time of unifocalization.
In patients who are deemed suitable for a single stage complete repair, a cardioplegia needle is placed in the ascending aorta and the aorta is cross-clamped. Cardioplegia is administered to achieve electromechanical silence. A right ventriculostomy is performed and the intra-cardiac anatomy inspected. The previously harvested autologous pericardium is trimmed to the appropriate size for VSD closure and sutured in place with 5-0 prolene. If there is an inter-atrial communication, this is closed through a right atriotomy incision. The aortic cross-clamp is then removed. During rewarming, the right ventricle to pulmonary artery conduit is sutured in place, performing the distal anastomosis first. The proximal anastomosis is usually augmented with an anterior patch to maintain the proper orientation of the homograft valve (Figure 3). All suture lines are inspected to assure reasonable hemostasis. Two transthoracic lines are placed, one in the left atrium and the other through right atrium into the right ventricle.
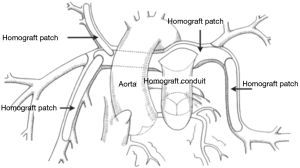
The patient is then weaned from cardiopulmonary bypass and the venous cannulas removed. We typically use epinephrine at 0.03 µg/kg/min, dopamine at 5 µg/kg/min, milrinone at 0.5 µg/kg/min, and CaCl at 20 µg/kg/min for cardiac support. A transesophageal echo is performed to inspect the ventricular function and integrity of the repair. Protamine is administered, the chest is packed with gauze while platelets and cryoprecipitate are administered. In recent years we have begun to routinely use factor eight inhibitor binding agent (FEIBA, Baxalta US Inc., Westlake Village, CA) at a dose of 10 units per Kg if the patient exhibits clinical signs of an ongoing coagulopathy. Chest tubes and temporary pacing wires are placed prior to chest closure.
Results and discussion
Midline unifocalization
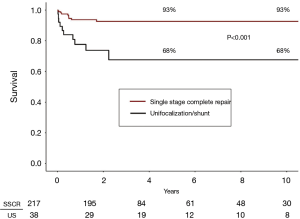
Over the past 16 years, we have performed 255 primary unifocalization procedures at our center (17,25). Of these 255 patients, 217 (85%) underwent a single stage complete repair. There were 3 operative deaths (1.5%) in the patients undergoing single stage complete repair. There were an additional eight late deaths subsequent to complete repair (Figure 4).
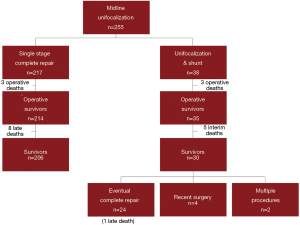
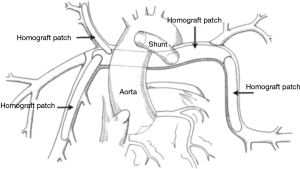
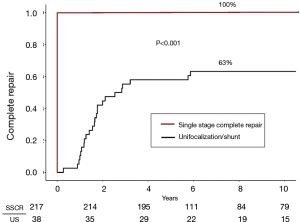
Thirty-eight patients underwent a unifocalization but were not deemed candidates for simultaneous VSD closure. These patients had a central shunt placed from the ascending aorta to unifocalized pulmonary artery bed (Figure 5). There were three operative deaths (8.8%) and five interim deaths in this cohort. Twenty-four of the 30 survivors have subsequently undergone complete repair, with one late death. The actuarial survival curves for these two cohorts is shown in Figure 6. The predicted survival was 93% vs. 68%, respectively (P<0.001). Nine-four percent of the patients who underwent a midline unifocalization have ultimately achieved complete repair. There was a significant disparity between those undergoing a single-stage complete repair vs. unifocalization/shunt (100% vs. 63% at 5 years, P<0.0001). The Kaplan-Meier curves for these two cohorts are shown in Figure 7.
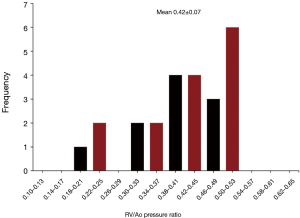
The average right ventricle to aortic peak systolic pressure ratio for patients undergoing a single-stage complete repair was 0.36±0.09, with a range of 0.20 to 0.58 (Figure 8). Patients who underwent and initial unifocalization/shunt and then complete repair had an average right ventricle to aortic pressure ratio of 0.42±0.07 (P<0.05 compared to single-stage complete repair). The data for these patients is shown in Figure 9.

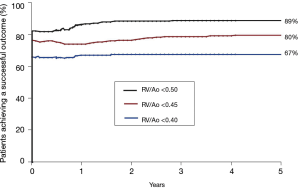
We recently performed an analysis of the likelihood of achieving an “ideal outcome” as defined by achieving all three of these measures of outcome including: (I) patient is alive, (II) patient has undergone complete repair, and (III) the RV/Ao pressure ratio is below a designated level. We selected three separate RV/Ao pressure ratio thresholds including 0.50, 0.45, and 0.40. The achievement of all three measures of outcome was 89%, 80%, and 67%, respectively (Figure 10). A review of the literature on the pulmonary artery rehabilitation approach reveals that the comparable percentages would be 20%, 5%, and 0%. Thus, combining measures of outcome widens the disparity between the midline unifocalization approach and the pulmonary artery rehabilitation approach (25,26).
Late hemodynamic follow-up
The majority of patients who undergo complete repair of PA/VSD/MAPCAs will have stable hemodynamics over time. In a study that evaluated 80 patients, the right ventricle to aortic pressure ratios were 0.39±0.09 as measured at the conclusion of a complete repair procedure and were 0.36±0.07 in the same patients assessed at the time of their right ventricle to pulmonary artery conduit replacement (27). It should be recognized that this cohort of 80 patients was a select group by virtue of the fact that they had undergone both a complete repair and subsequent conduit replacement.
Reoperations on the unifocalized bed
While the majority of patients do remarkably well following midline unifocalization, there is a subset of patients who demonstrate an increase in pulmonary artery pressures and subsequently require unifocalization revision. Most of the cases will manifest within the first year following surgery. This observation prompted us to perform a study in order to evaluate the risk factors for requiring unifocalization revision (28). The results demonstrated that there were three risk factors predisposing to the need for unifocalization revision, specifically: (I) unifocalization/shunt vs. complete repair, (II) higher RV/Ao pressure ratios, and (III) absence of central pulmonary arteries. While the first two risk factors were anticipated, the third was not expected, since absence of central pulmonary arteries was not identified as a risk factor at the time of midline unifocalization.
The surgical approach that we have developed for unifocalization revision is performed through a re-do median sternotomy. We attempt to do the majority of the dissection prior to heparinization and the institution of cardiopulmonary bypass. This can usually be accomplished completely on the right side and to a lesser extent on the left due to the presence of the conduit (Figure 11A). Once bypass has been instituted, we remove the existing right ventricle to pulmonary artery conduit and complete the dissection of the left pulmonary artery. The right and left pulmonary arteries are then divided to facilitate access to the lower lobe (Figure 11B).
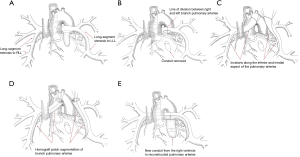
An incision is made along the inferior and medial aspect of the branch pulmonary artery (Figure 11C). This incision is continued into the medial lower lobe orifice. A homograft patch is then sutured in place to augment the lower lobe artery (Figure 11D). The operation is completed by augmenting the central branch pulmonary arteries and replacing the right ventricle to pulmonary artery conduit (Figure 11E). This technique has revolutionized our approach to unifocalization revision and we no longer perform thoracotomies for this clinical situation.
The results of unifocalization revision have recently been reported (29). This study demonstrated that patients who initially underwent a single stage complete repair fared much better than those who had undergone a unifocalization/shunt (Figure 12). Specifically, survival was 100% for single stage repair vs. 75% for unifocalization/shunt. In addition, 9% of patients required a second unifocalization revision in the single stage cohort vs. 25% for the unifocalization/shunt group. Collectively, 91% of the single stage repair cohort were alive and had not required a second revision vs. 56% for the unifocalization/shunt group (P<0.001). The hemodynamic results for unifocalization revision are summarized in Figure 13. The pulmonary artery to aortic pressure ratios decreased from 0.82±0.18 to 0.41±0.09 (P<0.001).


Conclusions
PA/VSD/MAPCAs is a complex form of congenital heart disease. While the intra-cardiac anatomy in PA/VSD/MAPCAs is remarkably uniform, there is a wide diversity of anatomy for both the pulmonary arteries and MAPCAs. This diverse anatomy provides a mandate for a surgical approach that is versatile and can address the many variations of this disease. The mid-line unifocalization approach is our choice for patients with predominantly single-supply MAPCAs and was used in the preponderance (80%) of the patients. It is our belief that the long-term outcomes for PA/VSD/MAPCAs will be determined by two factors; specifically, (I) the number of patients achieving complete repair, and (II) the achievement of low right ventricular pressures.
Acknowledgments
Figures 2, 3, 5, 11A,B,C,D,E were drawn by Erin Anne Mainwaring.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Raghav A. Murthy) for the series “Management of Congenital Heart Disease” published in Journal of Thoracic Disease. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: The series “Management of Congenital Heart Disease” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board (IRB) at Stanford University (Protocol ID 33924) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bull K, Somerville J, Spiegelhalter D. Presentation and attrition in complex pulmonary atresia. JACC 1995;25:491-9. [Crossref] [PubMed]
- Leonard H, Derrick G, O’Sullivan J, et al. Natural and unnatural history of pulmonary atresia. Heart 2000;84:499-503. [Crossref] [PubMed]
- Reddy VM, Liddicoat JR, Hanley FL. Midline one-stage unifocalization and repair of pulmonary atresia with ventricular septal defect and major aortopulmonary collaterals. J Thorac Cardiovasc Surg 1995;109:832-44. [Crossref] [PubMed]
- Carotti A, Albanese SB, Filleppelli S, et al. Determinants of outcome after surgical treatment of pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries. J Thorac Cardiovasc Surg 2010;140:1092-103. [Crossref] [PubMed]
- Barron DJ, Botha P. Approaches to pulmonary atresia with major aortopulmonary collateral arteries. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2018;21:64-74. [Crossref] [PubMed]
- Song SW, Park HK, Park YH, et al. Pulmonary atresia with ventricular septal defects and major aortopulmonary collateral arteries. Circ J 2009;73:516-22. [Crossref] [PubMed]
- Ishibashi N, Shin’oka T, Ishiyama M, et al. Clinical results of staged repair with complete unifocalization for pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries. Eur J Cardiothorac Surg 2007;32:202-8. [Crossref] [PubMed]
- Kim H, Sung SC, Choi KH, et al. A central shunt to rehabilitate diminutive pulmonary arteries in patients with pulmonary atresia with ventricular septal defect. J Thorac Cardiovasc Surg 2015;149:515-20. [Crossref] [PubMed]
- Mumtaz MA, Rosenthal G, Qureshi A, et al. Melbourne shunt promotes growth of diminutive central pulmonary arteries in patients with pulmonary atresia, ventricular septal defect, and systemic-to- pulmonary collateral arteries. Ann Thorac Surg 2008;85:2079-83. [Crossref] [PubMed]
- Hibino N, He D, Yuan F, et al. Growth of diminutive central pulmonary arteries after right ventricle to pulmonary artery homograft implantation. Ann Thorac Surg 2014;97:2129-33. [Crossref] [PubMed]
- Chen Q, Ma K, Hua Z, et al. Multistage pulmonary artery rehabilitation in patients with pulmonary atresia, ventricular septal defect and hypoplastic pulmonary artery. Eur J Cardiothorac Surg 2016;50:160-6. [Crossref] [PubMed]
- Zhang Y, Hua Z, Yang K, et al. Outcomes of the rehabilitative procedure for patients with pulmonary atresia, ventricular septal defect and hypoplastic pulmonary arteries beyond the infant period. Eur J Cardiothorac Surg 2014;46:297-303. [Crossref] [PubMed]
- Soquet J, Liava’a M, Eastaugh L, et al. Achievements and limitations of a strategy of rehabilitation of native pulmonary vessels in pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries. Ann Thorac Surg 2017;103:1519-26. [Crossref] [PubMed]
- Mainwaring RD, Reddy VM, Perry SB, et al. Late outcomes in patients undergoing aortopulmonary window for pulmonary atresia/stenosis and major aortopulmonary collaterals. Ann Thorac Surg 2012;94:842-8. [Crossref] [PubMed]
- Mainwaring RD, Rosenblatt TR, Patrick WL, et al. Surgical results for patients with pulmonary atresia and a ductus arteriosus or hemi-truncus to one lung and major aortopulmonary collateral arteries to the contralateral lung. Ann Thorac Surg 2018;106:568-74. [Crossref] [PubMed]
- Watanabe N, Mainwaring RD, Reddy VM, et al. Early complete repair of pulmonary atresia with ventricular septal defect and major aortopulmonary collaterals. Ann Thorac Surg 2014;97:909-15. [Crossref] [PubMed]
- Mainwaring RD, Patrick WL, Roth S, et al. Surgical algorithm and results for repair of pulmonary atresia/ventricular septal defect/major aortopulmonary collaterals. J Thorac Cardiovasc Surg 2018;156:1194-204. [Crossref] [PubMed]
- Greene CL, Mainwaring RD, Sidell D, et al. Impact of phrenic nerve palsy and the need for diaphragm plication following surgery for pulmonary atresia with ventricular septal defect and major aortopulmonary collaterals. Seminars in Thoracic and Cardiovascular Surgery 2018;30:318-24. [Crossref] [PubMed]
- Mainwaring RD, Patrick WL, Carrillo SA, et al. Prevalence and anatomy of retro-esophageal major aortopulmonary collateral arteries. Ann Thorac Surg 2016;102:877-82. [Crossref] [PubMed]
- Carrillo SA, Mainwaring RD, Patrick WL, et al. Surgical repair of pulmonary atresia/ventricular septal defect/major aortopulmonary collaterals with absent intra-pericardial pulmonary arteries. Ann Thorac Surg 2015;100:606-14. [Crossref] [PubMed]
- Mainwaring RD, Reddy VM, Hanley FL. Surgical reconstruction of pulmonary stenosis with ventricular septal defect and major aortopulmonary collaterals. Ann Thorac Surg 2013;95:1417-21. [Crossref] [PubMed]
- Mainwaring RD, Sheikh AY, Reddy VM, et al. Surgical results in patients with pulmonary atresia/major aortopulmonary collaterals and Alagille syndrome. Eur J Cardiothorac Surg 2012;42:235-40. [Crossref] [PubMed]
- Grosse-Wortmann L, Yoo SJ, van Arsdell G, et al. Preoperative total pulmonary blood flow predicts right ventricular pressure in patients early after complete repair of tetralogy of Fallot and pulmonary atresia with major aortopulmonary collateral arteries. J Thorac Cardiovasc Surg 2013;146:1185-90. [Crossref] [PubMed]
- Zhu J, Meza J, Kto A, et al. Pulmonary flow study predicts survival in pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries. J Thorac Cardiovasc Surg 2016;152:1494-503.e1. [Crossref] [PubMed]
- Mainwaring RD, Patrick WL, Rosenblatt TR, et al. Analysis of achieving an “ideal outcome” following midline unifocalization in pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries. Asian Cardiovasc Thorac Ann 2019;27:11-7. [Crossref] [PubMed]
- Mainwaring RD, Hanley FL. The earth is round! Letter to the Editor. J Thorac Cardiovasc Surg 2019;157:e207. [Crossref] [PubMed]
- Mainwaring RD, Reddy VM, Peng L, et al. Hemodynamic assessment after complete repair of pulmonary atresia/major aortopulmonary collaterals. Ann Thorac Surg 2013;95:1397-402. [Crossref] [PubMed]
- Mainwaring RD, Patrick WL, Ma M, et al. An Analysis of patients requiring unifocalization revision following midline unifocalization for pulmonary atresia with ventricular septal defect and major aortopulmonary collaterals. Eur J Cardiothorac Surg 2018;54:63-70. [Crossref] [PubMed]
- Mainwaring RD, Patrick WL, Rosenblatt TR, et al. Surgical results of unifocalization revision. J Thorac Cardiovasc Surg 2019;158:534-45.e1. [Crossref] [PubMed]


