
Preoperative computed tomography-guided pulmonary nodule localization augmented by laser angle guide assembly
Introduction
Lung cancer is the leading cause of cancer deaths internationally and is frequently diagnosed at late stages, which contributes to lung cancer’s high mortality rate (1,2). In recent years, low-dose CT screening has been proven to reduce lung cancer mortality and hence gained popularity (3,4). The small, sub-centimeter pulmonary nodules revealed by CT screening frequently require tissue removal for diagnosis and treatment, but these nodules are difficult to detect, either visually or by palpation during thoracoscopy. Therefore, there is a high demand for pre-operative localizations that render the nodules ‘visible’, which many studies have attempted to oblige (5-22).
Several methods have been reported for pre-operative localization, including electromagnetic navigation bronchoscopy (23), ultrasound-guided approaches (24,25), and CT-guided approaches (19). Preoperative CT-guided localizations (POCTGL) that utilize different marker materials have gained popularity in an attempt to make pulmonary nodules ‘visible’ during invasive procedures since sub-centimeter ground glass nodules are almost exclusively found in CT scans. The diagnostic or treatment procedure usually begins with the selection of a puncture point on the skin surface. Subsequently, a puncture route is created on the selected axial section of the CT scan, according to the planned depth and angle. With an “experienced hand”, the puncture is completed (18). To our knowledge, there is no set guideline for the “angle” of puncture. To aid in the precision of punctures, we have developed the Laser Angle Guide Assembly® (LAGA) as a reference for the puncture angle.
Even though thoracic medicine specialists rely on their past experience when performing localizations, most are unfamiliar with proper localization procedures. In this report, we share our experience of utilizing an exact laser guide in place of the “experienced hands” to handle POCTGL procedures.
Methods
Patients
Consecutive patients who received POCTGL for small (≤20 mm) pulmonary nodules between May 2015 and June 2018 at a tertiary medical referral center were prospectively included in this study. The electronic chart records and CT scans of localizations on the Picture Archiving and Communication System (PACS) were reviewed. This study was approved by the Institutional Review Board at the Chung Shan Medical University Hospital (No. CS 13221).
Localization procedures
The details of the procedures were published previously (26). Briefly, patients were fixed well in position with vacuumed bean-bags. All POCTGL procedures were performed under local anesthesia. After the puncture route was planned on the CT scans, the chosen axial level of the body and the target were aligned to the axial laser (red laser, Figure 1A), and the puncture angle was guided with another laser (green laser, Figure 1A) that was projected from the patient’s caudal side and consisted of the planned CT scan angle guided by the LAGA. Then, a 20-G, 90-mm spinal needle (Meditop, Thailand) with an inner solid needle and outer channel was used for the puncture, according to the planned depth controlled by a stopper (Figure 1B). Patent Blue Vital dye (PBV, Guerbet, France, 2.5%, 0.2–0.4 mL) was used to tattoo the tumor, as well as the visceral pleural surface when the nodule was less than 20 mm away from the visceral pleura. The inner needle was then withdrawn, the PBV injected with a 1-mL insulin syringe, and the inner needle placed back as soon as possible to prevent the atmospheric air from entering the body (Figure 1C). For those lesions deeper than 20 mm, a hookwire, cut 5–10 mm longer than the distance from the nodule to the visceral pleura, was implanted along with the PBV at the target nodule (Figure 1D). The hookwire was cut shorter and embedded in the body to prevent it from dropping out. The final CT scan was performed to confirm the accuracy of the localization. The standard localization was achieved when the PBV was in contact with the target nodule on the CT scan, which meant that the tip of the puncture needle should be within 5 mm of the target nodule.
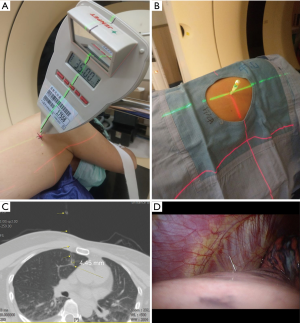
No oxygen supplementation or bed restriction was needed during the preoperative waiting period after the POCTGL.
Surgery
All of the surgeries were performed with Video-Assisted Thoracoscopic Surgery (VATS) under general anesthesia. The surgical procedure (either wedge resection, segmentectomy, or lobectomy) was selected according to a consensual principle: when a lesion was smaller than 10 mm and located at the periphery, wedge resection would be done; segmentectomy would be reserved for a centrally-located tumor; and for a solid nodule larger than 10 mm, lobectomy would be chosen. Some tiny nodules, such as 3-mm nodules, were resected because they were removed along with a main suspected lesion in the same surgery.
Data collection
We collected retrospective data on the following: patients’ age; gender; nodule size, location, and number; pathology; localization angle and depth; operation time; and complications. Data on the operation methods and hospital length of stay (LOS) were also collected.
During the operation, when the dye was injected from the mediastinum side, diaphragm side, or fissure side of the lung, the locations of the nodules were defined as mediastinum, diaphragm, or fissure, respectively. If the lesion was aimed at through a different lobe, such as through the right lower lobe to target a right upper lobe nodule, the location was defined as cross-lobe.
The time used for localization was defined as beginning from the first tomography and ending at the last checked CT scan.
A successful targeting procedure was defined as completion of the procedure in the CT room with the PBV contacting the lesion, whereas successful surgical field targeting was defined as when the PBV or hookwire were visible in the lung and the target nodules were found in the resected tattooed lung without PBV spillage, fading, or hookwire drop-out (19,27).
Complications were also evaluated. Pneumothorax was defined as any pneumothorax noted in the CT scan localization, even in trace amounts. A challenge was encountered when defining the lung parenchymal hemorrhage because it was difficult to differentiate between the injected dye and blood from the hemorrhage; however, from our daily CT-guided biopsy practice, we knew that parenchyma hemorrhage would cause hemoptysis, and therefore hemoptysis was recorded (28). Air embolus was defined as any radiolucent sign that was noted within the vascular system in the CT scan localization.
Statistics
The statistical analyses were performed with SPSS Statistics for Windows, version 18.0 (SPSS Inc., Chicago, Ill., USA). The categorical data were tested with the χ2 test; for data where the number in a cell was <5, Fisher’s exact test was used, and for ordinal data, the linear-by-linear association test was adopted. Continuous data were tested with an independent Student’s t-test. The multiple variable analysis was performed with backward binary logistic regression. The procedure time curve was drawn and tested by linear regression and analysis of variance.
Results
Between May 2015 and June 2018, 187 consecutive patients who received LAGA-assisted POCTGL were enrolled. Of these, 10 (5.9%) patients had COPD. None of the enrolled patients had active tuberculosis. Most of the patients were female (136, 72.2%) with an average age of 55.4±9.4 years. Data on smoking history were not available. A total of 196 surgeries were performed, including 9 with bilateral lesions. In 303 resected nodules, 266 were localized pre-operatively.
Localization
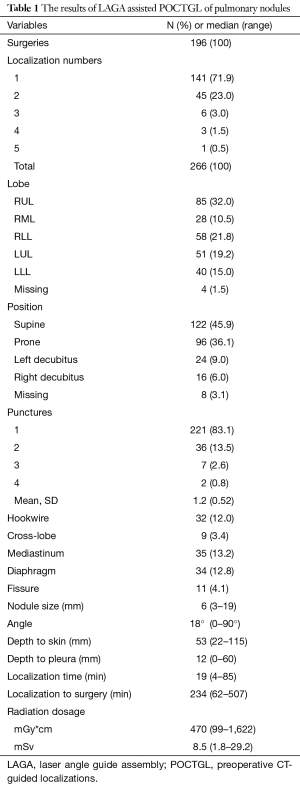
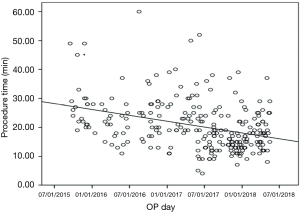
The number of nodules localized for one surgery was between 1 and 5, though 22.1% of the patients had more than 1 nodule (Table 1). Most of the nodules were located at the right upper lobe (RUL), followed by the left upper lobe (LUL). The proportions of nodules located adjacent to the mediastinum, diaphragm, and fissure were 13.2%, 12.8%, and 4.1%, respectively. The cross-lobe approach was chosen for 9 nodules. The median size of the nodules was 6 mm (mean ± standard deviation =6.3±2.9). The position of localization was mostly supine. A hookwire was inserted in 32 (12%) of the nodules. Most (83.1%) of the localizations were completed with a single puncture and 96.6% with two punctures. The median angle of approach was 18 degrees. The median nodule-to-skin and nodule-to-pleura depths were 53 and 12 mm, respectively, with maximums of 115 and 60 mm, respectively. The median time from dye injection to seeing the dye during surgery was about 4 hours, and after 8.5 hours the PBV could still be visualized clearly. The median time spent on localization was 19 minutes. The localization time significantly decreased, from 27 to 16 minutes, from 2015 to 2018 (Figure 2).
Full table
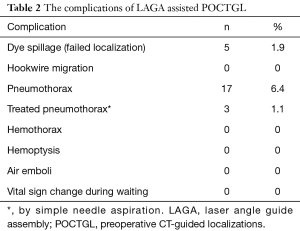
All of the 266 (100%) POCTGL procedures were completed satisfactorily. The successful targeting rate was 98.1% in the surgical field. There were 5 (1.9%) cases in which the PBV was not recognized within the lung with spillage to the pleura and was re-localized with the aid of the needle puncture holes on both the visceral and parietal pleural surfaces (Table 2). There were no hemothorax, air emboli, or hemoptysis complications among these POCTGL cases for the 266 nodules in 196 surgeries (Table 2). There were no vital sign changes, no need for intubation during the perioperative periods, and no hookwire migration.
Full table
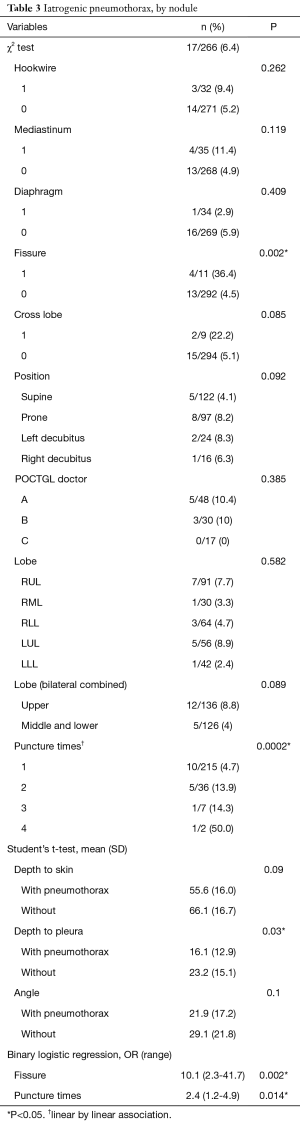
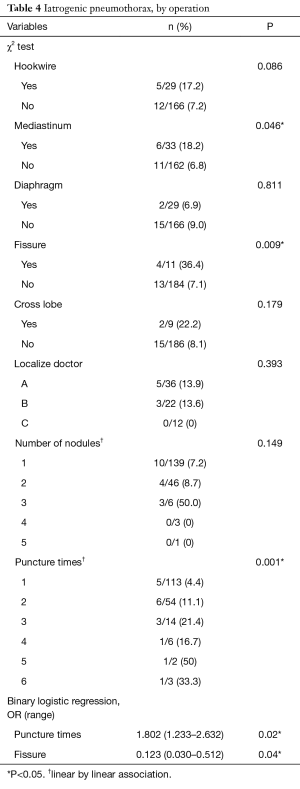
Pneumothorax was noted in 17 (6.4%) localizations and only 3 (1.1%) cases needed temporary treatments with aspiration, whereas the rest were closely followed. The risk factors associated with pneumothorax were analyzed (Table 3). Higher numbers of punctures per nodule, closer proximity of the tumor adjacent to the fissure, and shorter distances from the nodule to the visceral pleura were factors associated with pneumothorax in the univariable analysis. The multivariable analyses showed that the odds ratios for puncture times/nodule and tumors adjacent to the fissure were 2.4 (95% confidence interval, 1.2–4.9) and 10.1 (95% confidence interval, 2.3–41.7), respectively. The factors related to pneumothorax were also analyzed based on every single surgery (Table 4). The univariable analyses revealed that the puncture times/surgery and whether the tumor was adjacent to the fissure or mediastinum were contributing factors for pneumothorax. The multivariable analyses disclosed that an odds ratio of 1.8 (95% confidence interval, 1.2–2.6) for puncture times/surgery and an odds ratio of 8.1 (95% confidence interval, 1.9–33.3) for any nodule adjacent to the fissure were factors associated with pneumothorax.
Full table
Full table
Surgery
There were 196 thoracoscopic surgeries performed on 187 patients (Table 1). No conversion to thoracotomy was needed. There were 274 wedge resections and 21 anatomic resections. Some segmentectomy procedures were also inducted by the POCTGL. Some cases had anatomic resection and wedges in the same surgery. Multiple nodules may have been included in one resection. The median operative time was 105 minutes. The median hospital stay was 5 days. No 30-day mortality was reported.
Pathology
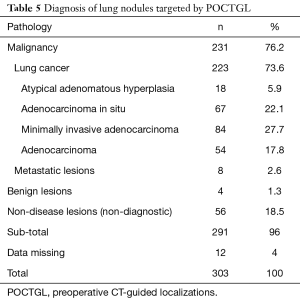
Of these 187 patients, 168 (89.8%) received surgical procedures that were diagnostic. There were 164 (87.7%) malignant and 4 benign lesions. Of the 303 pulmonary nodules, 223 (73.6%) were lung cancers (Table 5) and minimally invasive adenocarcinoma (27.7%) was the most frequent finding, followed by adenocarcinoma in situ (22.1%).
Full table
Discussion
With the increasing number of small pulmonary nodules found via CT screening programs, there is a greater need for pre-operative localizations. Depending on the expertise and availability of experts within the facility, thoracic medicine specialists, surgeons, and pulmonologists may be involved in the POCTGL of pulmonary nodules. LAGA® is an excellent angle reference to assist in performing these procedures. Alternatively, some surgeons have reported successful localizations with costlier navigation systems in a hybrid operating room setting (19).
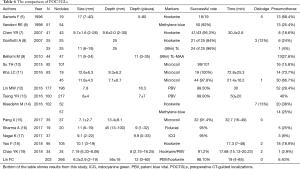
The performance of this series was compared to those of other published reports (Table 6) (5-18). The present investigation had the largest series with the smallest average nodule size. The procedure was rapid and accurate and the success rate was high with few cases of pneumothorax. Our series demonstrates one of the best POCTGL performances in the literature.
The definitions for successful targeting are still ambiguous (5-18). While most studies have defined successful targeting as the ability to place the markers successfully for surgical reference, the distance from the tumor to the markers remains ill-defined. Yao et al. considered a distance of 15 mm between the lesion and the localization needle as successful targeting (18). During the surgery, we considered the dye at a distance of 10 mm away from the nodule as too far and would likely miss the nodule. In contrast with methylene blue, PBV seldom spreads out more than 5 mm (Figure 1C), even when larger amounts are used (12). Placing the needle adjacent to the nodule was the only way to have the dye in close contact with the nodule; this was our operative definition of successful targeting because it ensured that when the PBV was resected within the safety margin (maximum diameter of the nodule) (29), the nodule would definitely be included. It not only obviated the need and pressure for the surgeon to visually identify the nodule in the resected specimen, but also alleviated the surgeon’s doubt regarding whether the nodule was really harvested.
The policy of localization with dye for surface nodules and a hookwire for deeper nodules is well accepted by surgeons (19); however, the high migration rate of the hookwire has been a major concern. The migration of the hookwire might result in missed targets and lead to complications (30). We adopted two safety measures to avoid failures of localization. First, we dual-marked the lesion with the PBV and a hookwire. Second, the hookwire was cut shorter and embedded in the body to prevent it from dropping out. As a result, no hookwire migration was observed among our 32 procedures. However, dual markings were only feasible in lesions far from the pleura.
A benefit of using PBV is that it stays in the lung for a long duration without spreading out. Our results suggested that PBV can stay in the lung tissue for more than 8 hours. Lin et al. also reported that PBV stayed in the lung for 561 minutes (12). Despite PBV’s long duration, when the needle did not penetrate deep enough into the lung tissue, the dye might still leak out. To prevent this, special attention was paid to ensure that the PBV, in addition to the needle, contacted the lung lesion in the final CT scan. We reviewed the failed cases and found that no dye was detected on the CT scan in these cases. Therefore, we would like to stress the importance of clearly identifying the PBV on the CT scan before the POCTGL is assessed as complete.
In our series, no hemothorax, hemoptysis, or air emboli complications were noted. Air emboli can be fatal and indicate communication between the pulmonary vessels with the atmosphere or airways (14,30). The precautious maneuver of cutting the hookwire shorter and hiding it in the chest wall prevented the atmospheric air from entering the pulmonary vessels. The LAGA further helped the hookwire to reach the planned destination without an accidental interchange between the airway and the pulmonary vessels. Similarly, hemothorax or hemoptysis (lung parenchymal hemorrhage) were avoided by planning the puncture pathway so that the major vessels were circumvented in order to prevent accidental penetrations.
Although pneumothorax could not be completely avoided, we achieved a pneumothorax incidence as low as 6.4% in our study, which is much lower than that reported in other series (Table 6). According to our statistical analysis, with every additional puncture, we doubled the risk of pneumothorax; this was also observed by Yao et al. (18). Repeated punctures over the adjacent visceral pleura induced pneumothorax. Under the assistance of LAGA, 71.9% of our POCTGL were achieved in one puncture and 94.9% were finalized within 2 punctures. Nevertheless, little information is available in the medical literature regarding the association between the number of punctures and complications. Our results showed that performing the POCTGL on nodules adjacent to the fissure had a greater risk of pneumothorax. For nodules adjacent to the fissure, the tip of the needle was inevitably pointed towards the fissure. We speculated that there was a greater chance that the needle would repeatedly touch the same fissure of the visceral pleura during breathing movements. Limited by our study design, these repeated punctures are not shown in our data collection. Unlike localization procedures performed under general anesthesia and positive airway pressure, where pneumothorax tends to happen more frequently (19), our procedures were performed under local anesthesia with negative airway pressure, and meticulously preventing the outside air from entering the chest cavity during the localization procedure was also key in preventing pneumothorax.
In addition to the decreased prevalence of procedure-related complications, our average procedure time was shorter than that of other studies (Table 6); (7,11-13,15,17). Interestingly, our first-year resident completed 5 LAGA-assisted POCTGL procedures, and there was a lack of associated complications, 100% targeting, and a median procedure time of 19 minutes (data not shown). Therefore, employing LAGA could improve the efficiency of POCTGL independent of the operator’s experience.
Full table
A distinct advantage of using LAGA was that it replaced the need for real-time fluoroscopy in POCTGL and thus reduced the medical staff’s radiation exposure. POCTGL is seldom performed by non-radiologists; however, since the introduction of low-dose CT lung cancer screening, the hybrid operating room and the electromagnetic navigation bronchoscopic system have gained popularity, and so localization may become a skill that thoracic surgeons and pulmonologists have the advantage of mastering (31,32).
A limitation of this study is that it is a one-arm, single-center study. A randomized trial that compares LAGA-aided POCTGL against other methods, such as the hybrid operating room and alternative methods of localization, and includes economic considerations may be warranted in the future.
Conclusions
LAGA enhanced the precision of POCTGL. It reduced the need for repeated penetrations, diminished the chance of pneumothorax and other complications, and increased the rate of targeting success and efficiency.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board at the Chung Shan Medical University Hospital (No. CS 13221).
References
- Barta JA, Powell CA, Wisnivesky JP. Global epidemiology of lung cancer. Ann Glob Health 2019;85:8. [Crossref] [PubMed]
- Pisani P, Parkin DM, Bray F, et al. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer 1999;83:18-29. [Crossref] [PubMed]
- Lin FC, Chen CY, Tsai SC. Lung Cancer Screening: Where we are and unanswered questions. SM J Pulm Med 2016;2:1024.
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. New Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Sartoris F, Cittadini G, Saitta S, et al. CT-guided needle localization of lung nodules for thoracoscopic resection. Eur Radiol 1996;6:420-4. [Crossref] [PubMed]
- Vandoni RE, Cuttat JF, Wicky S, et al. CT-guided methylene-blue labelling before thoracoscopic resection of pulmonary nodules. Eur J Cardiothorac Surg 1998;14:265-70. [Crossref] [PubMed]
- Chen YR, Yeow KM, Lee JY, et al. CT-guided hook wire localization of subpleural lung lesions for video-assisted thoracoscopic surgery (VATS). J Formos Med Assoc 2007;106:911-8. [Crossref] [PubMed]
- Gonfiotti A, Davini F, Vaggelli L, et al. Thoracoscopic localization techniques for patients with solitary pulmonary nodule: hookwire versus radio-guided surgery. Eur J Cardiothorac Surg 2007;32:843-7. [Crossref] [PubMed]
- Bellomi M, Veronesi G, Trifiro G, et al. Computed tomography-guided preoperative radiotracer localization of nonpalpable lung nodules. Ann Thorac Surg 2010;90:1759-64. [Crossref] [PubMed]
- Su TH, Fan YF, Jin L, et al. CT-guided localization of small pulmonary nodules using adjacent microcoil implantation prior to video-assisted thoracoscopic surgical resection. Eur Radiol 2015;25:2627-33. [Crossref] [PubMed]
- Kha LC, Hanneman K, Donahoe L, et al. Safety and efficacy of modified preoperative lung nodule microcoil localization without pleural marking: a pilot study. J Thorac Imaging 2016;31:15-22. [Crossref] [PubMed]
- Lin MW, Tseng YH, Lee YF, et al. Computed tomography-guided patent blue vital dye localization of pulmonary nodules in uniportal thoracoscopy. J Thorac Cardiovasc Surg 2016;152:535-44.e2. [Crossref] [PubMed]
- Tseng YH, Lee YF, Hsieh MS, et al. Preoperative computed tomography-guided dye injection to localize multiple lung nodules for video-assisted thoracoscopic surgery. J Thorac Dis 2016;8:S666-71. [Crossref] [PubMed]
- Kleedehn M, Kim DH, Lee FT, et al. Preoperative pulmonary nodule localization: a comparison of methylene blue and hookwire techniques. AJR Am J Roentgenol 2016;207:1334-9. [Crossref] [PubMed]
- Pang X, Xue L, Chen J, et al. A novel hybrid technique for localization of subcentimeter lung nodules. J Thorac Dis 2017;9:1107-12. [Crossref] [PubMed]
- Sharma A, McDermott S, Mathisen DJ, et al. Preoperative localization of lung nodules with fiducial markers: feasibility and technical considerations. Ann Thorac Surg 2017;103:1114-20. [Crossref] [PubMed]
- Nagai K, Kuriyama K, Inoue A, et al. Computed tomography-guided preoperative localization of small lung nodules with indocyanine green. Acta Radiol 2018;59:830-5. [Crossref] [PubMed]
- Yao F, Wang J, Yao J, et al. Reevaluation of the efficacy of preoperative computed tomography-guided hook wire localization: A retrospective analysis. Int J Surg 2018;51:24-30. [Crossref] [PubMed]
- Chao YK, Pan KT, Wen CT, et al. A comparison of efficacy and safety of preoperative versus intraoperative computed tomography-guided thoracoscopic lung resection. J Thorac Cardiovasc Surg 2018;156:1974-83.e1. [Crossref] [PubMed]
- Iguchi T, Hiraki T, Gobara H, et al. Transfissural route used for preoperative localization of small pulmonary lesions with a short hook wire and suture system. Cardiovasc Intervent Radiol 2015;38:222-6. [Crossref] [PubMed]
- Miura H, Yamagami T, Tanaka O, et al. CT findings after lipiodol marking performed before video-assisted thoracoscopic surgery for small pulmonary nodules. Acta Radiol 2016;57:303-10. [Crossref] [PubMed]
- Imperatori A, Fontana F, Dominioni L, et al. Video-assisted thoracoscopic resection of lung nodules localized with a hydrogel plug. Interact Cardiovasc Thorac Surg 2019;29:137-43. [Crossref] [PubMed]
- Chen A, Pastis N, Furukawa B, et al. The effect of respiratory motion on pulmonary nodule location during electromagnetic navigation bronchoscopy. Chest 2015;147:1275-81. [Crossref] [PubMed]
- Nakano N, Miyauchi K, Imagawa H, et al. Immediate localization using ultrasound-guided hookwire marking of peripheral lung tumors in the operating room. Interact Cardiovasc Thorac Surg 2004;3:104-6. [Crossref] [PubMed]
- Kondo R, Yoshida K, Hamanaka K, et al. Intraoperative ultrasonographic localization of pulmonary ground-glass opacities. J Thorac Cardiovasc Surg 2009;138:837-42. [Crossref] [PubMed]
- Lin FC, Tsai SC, Tu HT, et al. Computed tomography-guided localization with laser angle guide for thoracic procedures. J Thorac Dis 2018;10:3824-8. [Crossref] [PubMed]
- Park CH, Han K, Hur J, et al. Comparative effectiveness and safety of preoperative lung localization for pulmonary nodules: a systematic review and meta-analysis. Chest 2017;151:316-28. [Crossref] [PubMed]
- Tomiyama N, Yasuhara Y, Nakajima Y, et al. CT-guided needle biopsy of lung lesions: a survey of severe complication based on 9783 biopsies in Japan. Eur J Radiol 2006;59:60-4. [Crossref] [PubMed]
- Sawabata N, Ohta M, Matsumura A, et al. Optimal distance of malignant negative margin in excision of non-small cell lung cancer: a multicenter prospective study. Ann Thorac Surg 2004;77:415-20. [Crossref] [PubMed]
- Sakiyama S, Kondo K, Matsuoka H, et al. Fatal air embolism during computed tomography–guided pulmonary marking with a hook-type marker. J Thorac Cardiovasc Surg 2003;126:1207-9. [Crossref] [PubMed]
- Bolton WD, Cochran T, Ben-Or S, et al. Electromagnetic navigational bronchoscopy reduces the time required for localization and resection of lung nodules. Innovations (Phila) 2017;12:333-7. [Crossref] [PubMed]
- Awais O, Reidy MR, Mehta K, et al. Electromagnetic navigation bronchoscopy-guided dye marking for thoracoscopic resection of pulmonary nodules. Ann Thorac Surg 2016;102:223-9. [Crossref] [PubMed]


