
Relationship of soluble ST2 to pulmonary hypertension severity in patients undergoing cardiac resynchronization therapy
Introduction
Cardiac resynchronization therapy (CRT) is an adjuvant treatment for refractory heart failure (HF) that may reverse left ventricular remodeling, improve symptoms, and reduce mortality (1-3). However, approximately one third of patients fail to show a benefit (4,5). Chronically elevated filling pressure from left HF can induce secondary pulmonary hypertension (PH), leading to increased morbidity and mortality. Identification of PH derived from echocardiography is an adverse prognostic marker in HF and for patients undergoing CRT. Serial assessment of PH after CRT can also be helpful, and improvement of PH after CRT has been identified as an independent positive prognostic marker (6-13). Soluble ST2 (sST2) is an emerging cardiac biomarker, expressed by strained cardiomyocytes, and linked to cardiac fibrosis in HF. Elevated concentrations of sST2 are associated with progressive myocardial remodeling and adverse HF prognosis (14-17). Serial measurements of sST2 provide incremental value to baseline levels, reflecting changes in myocardial remodeling overtime (16). Along with other biomarkers, sST2 concentrations before CRT implantation may help to predict clinical non-response and MACE after CRT (18,19). Besides being associated with left ventricular remodeling, concentrations of sST2 have been linked to pulmonary vascular diseases, including PH. Accordingly in this study, we aim to examine a biomarker panel of myocardial fibrosis (sST2, galectin-3), myocardial wall stretch [amino terminal pro-brain natriuretic peptide (NT-proBNP)], and myocardial necrosis [high-sensitivity troponin-I (hsTnI)] and examine its relationship with PH defined by right ventricular systolic pressure (RVSP) by Echo and changes in the biomarker values to changes in PH status. In a focus analysis with sST2, we hypothesized that baseline and serial sST2 measurements would correlate with PH in patients undergoing CRT and parallel PH changes after CRT. We also hypothesized that sST2 serial variation after CRT will be associated with clinical outcomes.
Methods
Study population and protocol
The “Biomarkers to Predict CRT Response in Patients With HF” (BIOCRT; Clinical Trials.gov # NCT01949246) trial is a prospective observational study of CRT at a single tertiary care center. Study design, inclusion and exclusion criteria were previously reported (20). In brief, eligible patients were CRT candidates ≥18 years old, NYHA functional class II–IV on optimal drug therapy, left ventricular ejection fraction ≤35%, QRS interval ≥120 ms and a HF exacerbation within the past year. Exclusion criteria included life expectancy less than 6 months, severe aortic stenosis, recent cardiac surgery or coronary revascularization, intermittent or continuous intravenous therapy, chronic obstructive pulmonary disease, primary PH and pregnancy.
A total of 111 patients were included in this analysis. Study participants were followed through a time horizon up to 2 years. Thirty two patients did not have RVSP at 6 months (12 participants completed the 6-month office visit, but did not have an echocardiogram, 15 participants had 6-month echocardiography, but RVSP was not measurable, 4 participants had MACE with 3 deaths and 1 hospitalization, 1 participant did not have 6-month follow up, but had follow up later and was noted to be MACE-free and have a positive CRT response), therefore, follow-up echocardiographic data was available in 79 patients. Of the 111 patients included in the study, 88 patients had baseline serum blood samples drawn and 78 patients had plasma samples. For the first 10 patients, we did not have plasma samples as we initially started collecting only serum samples. Some patients had a clinical follow-up but declined the serial blood draws. We had approximately 50% retention rate for follow-up serial samples over 6 months, with resulting 42 patients with serial serum samples and 35 patients with plasma. The institutional review board approved the study protocol and all patients provided written consent prior to study initiation.
Echocardiography
Echocardiographic measurements included end-systolic and end-diastolic volumes by the modified biplane Simpson’s method with computation of left ventricle ejection fraction (21,22). Right ventricular size and function were assessed by level 3 readers integrating all available parameters for each exam (RV diameters, tricuspid annular plane excursion, peak tricuspid annular velocity, fractional area change, myocardial performance index) (23). Tricuspid regurgitation was graded using American Society of Echocardiography (ASE) guidelines by level 3 readers, integrating vena contracta, proximal flow convergence, hepatic vein flow Doppler and jet area (24). RVSP was estimated using simplified Bernoulli equation on peak tricuspid regurgitation jet velocity by continuous wave Doppler (23).
RVSP definitions and serial assessment
PH was defined as RVSP greater than 35 mmHg (25,26). Mild-moderate PH was defined as RVSP >35 and ≤60 mmHg, and severe PH as RVSP greater than 60 mmHg. At 6-months, echocardiography was performed to assess change in RVSP and PH severity. Delta-RVSP classes were defined by change in PH severity class from baseline to 6 months post implantation. Progressors were those whose RVSP worsened by one or more classes from pre-implantation, while regressors are those whose PH severity improved by one or more classes. Persistent PH was defined as those with stable PH severity at 6 months from baseline.
Outcomes
Clinical endpoints included 6-month CRT response and 2-year MACE. CRT response was measured using the Heart Failure Clinical Composite Score (HF CCS). The HF CCS includes subjective metrics like NYHA functional class and global assessment score as well as objective measures of decompensation such as hospitalization and mortality (27). HF CCS scores that were stable or worsened were considered non-responders, while responders improved their HF CCS scores. Major adverse cardiovascular event (MACE) was defined as the composite endpoint of death, cardiac transplant, left ventricular assist device, and HF hospitalization. CRT response and MACE was adjudicated by a committee consisting of 2 cardiologists, blinded to the biomarker and echocardiography results. Disagreement was resolved by consensus with a third cardiologist.
Blood samples
Peripheral venous blood samples were obtained at the time of device implantation and during the 6-month follow up visit. Samples were stored at −80 °C and sent to independent laboratories for analysis. The laboratories were blinded to the clinical history and timing of the samples. Plasma sST2 concentrations (Presage ST2; Critical Diagnostics, San Diego, CA, USA) were measured using enzyme-linked immunosorbent assay with an interassay coefficients of variation (CV) <12% and intraassay CV of 2.3%. Plasma gal-3 measurements (BGM Galectin-3, BG Medicine, Inc.) were performed by enzyme linked immunosorbent assay (ELISA), with interassay CV of 2.2%, and intra-assay CV of 3.0%. Serum NT-proBNP measurements (Dimension Vista Flex, Siemens) were performed by a one-step sandwich chemiluminescent immunoassay, with interassay CV of <3% and intra-assay CV of <4%. Serum hs-TnI measurements (Dimension Vista; Siemens) were performed by the one-step sandwich chemiluminescent immunoassay had a total imprecision (CV) of 8.5% at 4.4 pg/mL and 4.6% at 11.8 pg/mL.
Statistical analysis
Descriptive statistics were presented as mean ± standard deviation (SD) for normally distributed variables or as a median with interquartile range for non-normal, continuous variables. Categorical variables were summarized with frequencies and percentages. Wilcoxon-rank-sum tests or t-tests were used to compare continuous variables and Fisher’s exact or chi-squared tests were used to compare categorical variables. Kruskal-Wallis test was used for >2 group comparison. Log transformation was applied for non-normally distributed continuous data. Two-year MACE-free survival was analyzed using Kaplan-Meier methodology and comparisons were made using a stratified log-rank test. We used logistic regression to test the association between CRT non-response and baseline log-transformed Echo metrics, 3-caterory PH by RVSP, and progressor categories. We used Cox proportional hazard models to test the association between MACE and baseline log-transformed Echo metrics, 3-caterory PH by RVSP, and progressor categories. A two-tailed P value of <0.05 was considered statistically significant. SAS (version 9.4; SAS Institute Inc., Cary, NC, USA) was utilized for all statistical analyses.
Results
Baseline clinical and echocardiography characteristics are displayed in Table 1. Baseline clinical and demographic characteristics did not differ amongst those with and without follow-up 6-month echocardiography (Table S1, all P=NS). There were 40 (36%) CRT non-responders and 31 (28%) participants had MACE.
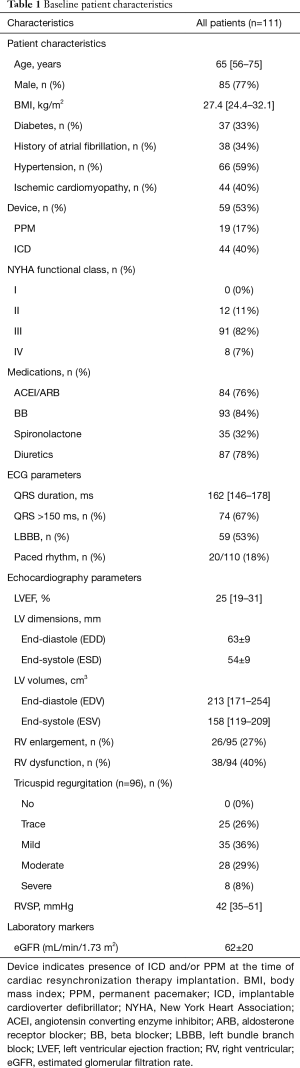
Full table
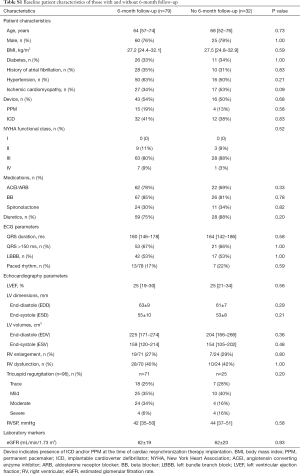
Full table
RVSP
Echo-based PH with RVSP >35 mmHg was present in 89 patients at baseline (80%), with a median RVSP in the study population of 42 mmHg (IQR, 29–60 mmHg). Severe PH with >60 mmHg was present in 13 (15%) patients. Of the baseline Echo parameters, RVSP was the strongest predictor of clinical events, and independently associated in multivariable models (Table 2). Compared to those with normal RVSP, severe PH was associated with greater than 5-fold increase risk of CRT non-response and two-year MACE (Table 3, Figure 1). Mild-moderate PH (RVSP 35–60 mmHg) had a slightly increased risk of CRT non-response and 2-year MACE compared to normal RVSP, but was not statistically significant.
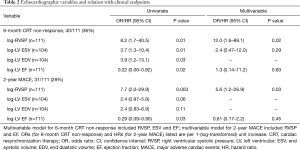
Full table

Full table
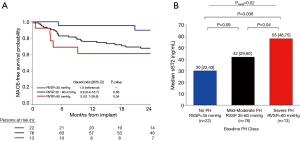
Serial RVSP and clinical outcomes
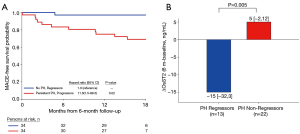
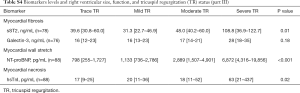
Table 4 depicts the association of clinical outcomes with changes in PH severity. RVSP progressors (those whose PH severity class worsened) and those with persistent PH had over 2-fold increase odds for being a CRT non-responder at 6 months and over 11-fold increase risk of MACE at 2-year (Table 4, Figure 2).

Full table
PH status by RVSP and biomarker levels
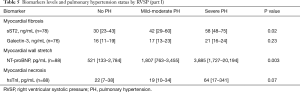
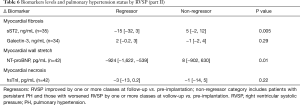
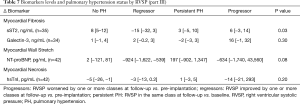
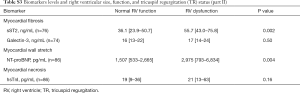
Tables 5-7 show the biomarker panel of myocardial fibrosis (sST2, galectin-3), myocardial wall stretch (NT-proBNP), and myocardial necrosis (hsTnI) and their relationship with PH status by RVSP. Of these biomarkers, only NT-proBNP and sST2 were discernable between groups. Both markers were also associated with right ventricular size/function and TR severity (Tables S2-S4).
Full table
Full table
Full table

Full table
Full table
Full table
The baseline median NT-proBNP levels had a graded increase based on PH severity by RVSP (P=0.003) and had lower values in regressors vs. non-regressors (P=0.01), there was no significant pattern of NT-proBNP changes at 6 months in those with no PH, regressor, persistent PH, and progressor (P=0.08).
For sST2, there was a graded increase in baseline median sST2 levels and PH severity by RVSP (P=0.02). Log-transformed baseline sST2 was associated with CRT non-response [OR 2.9 (1.1–7.6), P=0.03] and for MACE [HR 2.3 (1.02–5.3), P=0.04]. Lastly, when examining serial sST2 concentrations at 6 months, PH regressors had a median reduction in sST2 levels by 15 ng/mL, while non-regressors had increase of 5 ng/mL (P=0.005). Additionally, the sST2 changes at 6 months in those with no PH, persistent PH, progressor increased slightly (3–8 ng/mL) while the regressor group had a reduction in sST2 levels of 15 ng/mL, (P=0.03).
Discussion
In this study, we found that Echo-based RVSP was associated with 6-month CRT nonresponse and 2-year MACE. Severe PH patients were more likely to be CRT non-responder and have 2-year MACE than non-PH patients. Amongst the panel of 4 HF biomarkers of myocardial fibrosis, myocardial wall stretch, and myocardial necrosis, we observed a graded increase in baseline median NT-proBNP and sST2 concentrations with worsening PH severity. Progressors and those with persistent PH were more likely to be CRT non-responder at 6 months and have MACE at 2-years when compared to patients with no PH or regressors. Additionally, we found that PH regressors had a reduction in sST2 concentrations while those with no PH, persistent PH, and progressors had a slight increase in sST2 by 6 months. We did not observe this difference for NT-proBNP.
The present study provides further evidence that increased PH is related to morbidity and mortality in patients undergoing CRT (6,13). PH was a common finding in our population, occurring in 80% of our cohort, and was a strong independent predictor of 6-month CRT non-response and two-year MACE. PH in this population is caused by elevated left atrial pressure, which is the result of the variable combination of systolic dysfunction, diastolic dysfunction and/or associated valvular disease. Our finding that that PH (reflecting the global impact of these individual parameters) better predicted clinical outcomes than LVEF or LV size is not surprising. Pre-implantation RVSP measurement serves as a valuable independent prognostic marker beyond the severity of left heart systolic dysfunction.
While the observed relationship between myocardial wall stretch marker of NT-proBNP and RVSP PH severity is known and expected, we also found that sST2 and its serial changes better paralleled PH severity and progression/regression. While both markers can be influenced by volume status, our data suggest that sST2 may be more specific to PH. Interestingly; recent data suggest that extra-myocardial production of sST2 may account for some circulating concentrations of the marker (28). In the BIOCRT study, we found no trans-myocardial gradient when measuring sST2 concentrations in the coronary sinus (20), and other work demonstrated increased sST2 in pulmonary arterial endothelial cells (29), suggesting a potential implication for the pulmonary vasculature in sST2 release. Furthermore in patients with severe pulmonary inflammation, such as those with acute respiratory distress syndrome—a state of severe pulmonary vascular injury and inflammation—some of the highest reported concentrations of sST2 have been reported (30). Taken together, these findings suggest a pulmonary source for sST2 and could explain in part the relationship between serial sST2 and RVSP that we have found.
Study limitations
The study population reflects a small cohort of patients undergoing CRT at a single tertiary care center that may represent selection and treatment bias limiting generalizability. Our study population was predominantly male, had mild-moderate renal insufficiency, and had significant left ventricular systolic dysfunction characterized by primarily NYHA functional class III disease. We do not have inferior vena cava measurements, thus our data on RVSP does not include estimated right atrial pressure from inferior vena cava collapsibility. The small sample size limits the power to examine subgroups and produced large confidence intervals, which limited the number of adjustments in the multivariable model. Follow-up echocardiography and biomarker levels were not available in a proportion of patients at follow-up. Direct invasive measurements would have been interesting; however, this echo-based measure still correlated well with clinical outcomes.
Conclusions
Concentrations of sST2 are associated with PH severity in CRT patients. Reduction in sST2 concentrations is associated with PH regressors in these patients, which suggests its potential role to monitor PH after CRT. Our findings suggest this cardiovascular stress marker may contribute to the understanding of interplay between PH and adverse outcomes in CRT patients.
Acknowledgments
Funding: The study was supported by NIH/NHLBI K23HL098370. Dr. Truong also received support from the NIH L30HL093896. Dr Beaudoin is funded by Fonds de Recherche Québec-Santé and Heart and Stroke Foundation of Canada. The reagents/assays were provided and performed by Critical Diagnostics, BG Medicine, and Siemens Healthcare Diagnostics Inc.
Footnote
Conflicts of Interest: Dr. Januzzi is supported in part by the Hutter Family Professorship, receives grant support from Siemens, Singulex, Prevencio, and Roche, and has served as a consultant to Roche Diagnostics, Critical Diagnostics, Abbott, GE, Amgen, and Novartis. Dr. Singh receives grant support from St. Jude Medical, Medtronic Inc., Boston Scientific Corp., Sorin Group, Biotronik, BG Medicine and Siemens. Dr. Truong received grant support from Ziosoft, Inc. (formerly Qi Imaging LLC). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The institutional review board approved the study protocol and all patients provided written consent prior to study initiation.
References
- Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med 2009;361:1329-38. [Crossref] [PubMed]
- Tang AS, Wells GA, Talajic M, et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med 2010;363:2385-95. [Crossref] [PubMed]
- Chen Y, Duan C, Liu F, et al. Impact of etiology on the outcomes in heart failure patients treated with cardiac resynchronization therapy: a meta-analysis. PLoS One 2014;9:e94614. [Crossref] [PubMed]
- Yao G, Freemantle N, Calvert MJ, et al. The long-term cost-effectiveness of cardiac resynchronization therapy with or without an implantable cardioverter-defibrillator. Eur Heart J 2007;28:42-51. [Crossref] [PubMed]
- Bax JJ, Abraham T, Barold SS, et al. Cardiac resynchronization therapy: Part 2--issues during and after device implantation and unresolved questions. J Am Coll Cardiol 2005;46:2168-82. [Crossref] [PubMed]
- Wang J, Su Y, Bai J, et al. Elevated pulmonary artery pressure predicts poor outcome after cardiac resynchronization therapy. J Interv Card Electrophysiol 2014;40:171-8. [Crossref] [PubMed]
- Stern J, Heist EK, Murray L, et al. Elevated estimated pulmonary artery systolic pressure is associated with an adverse clinical outcome in patients receiving cardiac resynchronization therapy. Pacing Clin Electrophysiol 2007;30:603-7. [Crossref] [PubMed]
- Tedrow UB, Kramer DB, Stevenson LW, et al. Relation of right ventricular peak systolic pressure to major adverse events in patients undergoing cardiac resynchronization therapy. Am J Cardiol 2006;97:1737-40. [Crossref] [PubMed]
- Chatterjee NA, Upadhyay GA, Singal G, et al. Pre-capillary pulmonary hypertension and right ventricular dilation predict clinical outcome in cardiac resynchronization therapy. JACC Heart Fail 2014;2:230-7. [Crossref] [PubMed]
- Healey JS, Davies RA, Tang AS. Improvement of apparently fixed pulmonary hypertension with cardiac resynchronization therapy. J Heart Lung Transplant 2004;23:650-2. [Crossref] [PubMed]
- Bleeker GB, Schalij MJ, Nihoyannopoulos P, et al. Left ventricular dyssynchrony predicts right ventricular remodeling after cardiac resynchronization therapy. J Am Coll Cardiol 2005;46:2264-9. [Crossref] [PubMed]
- Rajagopalan N, Suffoletto MS, Tanabe M, et al. Right ventricular function following cardiac resynchronization therapy. Am J Cardiol 2007;100:1434-6. [Crossref] [PubMed]
- Shalaby A, Voigt A, El-Saed A, et al. Usefulness of pulmonary artery pressure by echocardiography to predict outcome in patients receiving cardiac resynchronization therapy heart failure. Am J Cardiol 2008;101:238-41. [Crossref] [PubMed]
- Shah RV, Chen-Tournoux AA, Picard MH, et al. Serum levels of the interleukin-1 receptor family member ST2, cardiac structure and function, and long-term mortality in patients with acute dyspnea. Circ Heart Fail 2009;2:311-9. [Crossref] [PubMed]
- Aimo A, Vergaro G, Ripoli A, et al. Meta-Analysis of Soluble Suppression of Tumorigenicity-2 and Prognosis in Acute Heart Failure. JACC Heart Fail 2017;5:287-96. [Crossref] [PubMed]
- Gaggin HK, Szymonifka J, Bhardwaj A, et al. Head-to-head comparison of serial soluble ST2, growth differentiation factor-15, and highly-sensitive troponin T measurements in patients with chronic heart failure. JACC Heart Fail 2014;2:65-72. [Crossref] [PubMed]
- Lupon J, Sanders-van Wijk S, Januzzi JL, et al. Prediction of survival and magnitude of reverse remodeling using the ST2-R2 score in heart failure: A multicenter study. Int J Cardiol 2016;204:242-7. [Crossref] [PubMed]
- Truong QA, Januzzi JL, Szymonifka J, et al. Coronary sinus biomarker sampling compared to peripheral venous blood for predicting outcomes in patients with severe heart failure undergoing cardiac resynchronization therapy: the BIOCRT study. Heart Rhythm 2014;11:2167-75. [Crossref] [PubMed]
- Skali H, Gerwien R, Meyer TE, et al. Soluble ST2 and Risk of Arrhythmias, Heart Failure, or Death in Patients with Mildly Symptomatic Heart Failure: Results from MADIT-CRT. J Cardiovasc Transl Res 2016;9:421-8. [Crossref] [PubMed]
- Goehler A, McMahon PM, Lumish HS, et al. Cost-Effectiveness of Follow-Up of Pulmonary Nodules Incidentally Detected on Cardiac CT Angiography in Patients with Suspected Coronary Artery Disease. Circulation 2014. [Crossref]
- Schiller NB, Acquatella H, Ports TA, et al. Left ventricular volume from paired biplane two-dimensional echocardiography. Circulation 1979;60:547-55. [Crossref] [PubMed]
- Folland ED, Parisi AF, Moynihan PF, et al. Assessment of left ventricular ejection fraction and volumes by real-time, two-dimensional echocardiography. A comparison of cineangiographic and radionuclide techniques. Circulation 1979;60:760-6. [Crossref] [PubMed]
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685-713. [Crossref] [PubMed]
- Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017;30:303-71. [Crossref] [PubMed]
- McQuillan BM, Picard MH, Leavitt M, et al. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation 2001;104:2797-802. [Crossref] [PubMed]
- Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015;46:903-75. [Crossref] [PubMed]
- Packer M. Proposal for a new clinical end point to evaluate the efficacy of drugs and devices in the treatment of chronic heart failure. J Card Fail 2001;7:176-82. [Crossref] [PubMed]
- Pascual-Figal DA, Perez-Martinez MT, Asensio-Lopez MC, et al. Pulmonary Production of Soluble ST2 in Heart Failure. Circ Heart Fail 2018;11:e005488. [Crossref] [PubMed]
- Shao D, Perros F, Caramori G, et al. Nuclear IL-33 regulates soluble ST2 receptor and IL-6 expression in primary human arterial endothelial cells and is decreased in idiopathic pulmonary arterial hypertension. Biochem Biophys Res Commun 2014;451:8-14. [Crossref] [PubMed]
- Bajwa EK, Volk JA, Christiani DC, et al. Prognostic and diagnostic value of plasma soluble suppression of tumorigenicity-2 concentrations in acute respiratory distress syndrome. Crit Care Med 2013;41:2521-31. [Crossref] [PubMed]


