
Electronic nicotine delivery systems (ENDS): not still ready to put on END
Introduction
Cigarette smoking is one of the most dangerous killer, with about 5 million deaths every year worldwide, accounting for 12% of all deaths among adults. According to World Health Organization (WHO) estimates, we are witnessing approximately 10 deaths due to smoking by the minute. Considering the total of deaths from cardiovascular and respiratory diseases, tobacco smoking is the cause in 10% and 36%, respectively. Among cancers, tobacco smoking is responsible for over 71% of all lung cancer deaths and 22% of all cancer deaths (1). In recent years, 15% of relative reduction in the smoking rates was reported worldwide; specifically, from 22.5% to 19.2% of smoking prevalence over 10 years (from 37.1% to 32.7% among men and from 8.0% to 5.8% among women). In high-income countries, the trend toward a decrease in smoking prevalence is more significant, but the rate of women smokers (16.4%) remains very high compared with the average rate (2).
The attitudes of people towards tobacco products has changed with the introduction of vaping devices. The first e-cigarettes (E-CIGs) had a design similar to usual cigarettes. They were cigarette-like, in order to keep the smoker's rituals intact. In this way, the consumer had the same feeling of smoking a normal cigarette. Subsequently, new-generation E-CIGs were introduced, with a greater number of accessories to meet the needs of the users. The new E-CIGs have several additional properties, such as recharging the battery, increasing the capacity of the reservoir, increasing concentration of steam and dispensing different concentration of chemical compounds through the variation of puff flow rate and duration.
Electronic nicotine delivery systems (ENDS) and electronic non-nicotine delivery systems (ENNDS) are usually formed by atomizer, battery, drip tip and reservoir. The reservoir is designed to contain an e-liquid usually constituted by nicotine, flavor concentrates and propylene glycol or glycerol. When the user activates the heating element, the liquid contained in the reservoir is vaporized, releasing an aerosol which contains a lot of substances (3,4).
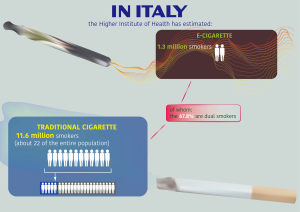
The global ENDS global market reached a value of US$11.5 billion in 2018 and is expected to gain US$26.84 billion by 2023 (5,6). About 56% regards US market and another 21% among be China, Italy, France, Poland and Germany (3–7% each) (7). In Italy, the Higher Institute of Health has estimated 11.6 million smokers (about 22 of the entire population) in 2018. In addition, the Higher Institute of Health has estimated 1.3 million e-cigarette smokers, of whom the 67.8% are dual smokers (Figure 1).
ENDS global policies
ENDS have recently become the center of a heated debate. On one hand, ENDS are deemed as an alternative to cigarettes and as benefit for smokers in their attempts to quit tobacco consumption, but on the other hand several concerns have been rising about risk on public health derived from ENDS use (Table 1). Moreover, it is not clear to what extend ENDS could impact in the harms of second-hand smoke and renormalization effect. ENDS are also seen as a possible threat for smoke-free policies. Because of the lack of unequivocal evidence on this topic, jurisdiction on ENDS is quite challenging.

Full table
So far, a broad range of policies approaches have been applied to ENDS worldwide and the policy around E-CIGs is rapidly evolving. The WHO Framework Convention on Tobacco Control (FCTC) has made considerable efforts over the last decade to identify best practices in product classification, provide policy domains and make adequate regulatory mechanisms and update regulations taking into account scientific developments on health warnings (8-11).
Product classification
Several countries have recognized a clear categorization of ENDS, within the existing laws, as the first step towards ENDS regulation. So far, countries have classified ENDS in seven categories: (I) tobacco products; (II) products imitating tobacco; (III) medicinal products; (IV) pharmaceutical products; (V) consumer products; (VI) poison; or (VII) ENDS (8).
Policy domains
In 2014, WHO presented a report on ENDS at the Sixth session of the Conference of the Parties (COP) which included scientific recommendations on ENDS. These indications were conceived after a survey on tobacco products in which more than half of the 90 participating Member States did not regulate ENDS. Several policy domains were included in the report: ban the use and sale of E-CIGs wherever the use and sale of conventional cigarettes are prohibited, apply the same marketing restrictions to E-CIGs as are applied to conventional cigarettes, ban companies from making claims regarding tobacco-use cessation (9). Subsequently, in 2016, WHO released a report (FCTC/COP/7/11) to COP. At first, the document has provided updated evidence of the health impact of ENDS and ENNDS and their potential role in tobacco cessation and tobacco control efforts; secondly, it has also specified a non-exhaustive list of regulatory options that Parties might consider classified by four objectives (prevent the initiation of ENDS by non-smokers and youth; minimize as far as possible potential health risks for ENDS users and protect non-users from exposure to their emissions; prevent unproven health claims made about ENDS; protect tobacco control activities from all commercial and other interests related to ENDS). Regulatory options included: ban the sale and possession of ENDS to minors, ban advertising of ENDS, regulate places of sales; ban the use of flavors appealing to minors; provide appropriate labelling on devices and e-liquids; prohibit the use of ENDS in indoor spaces; prohibit implicit and explicit claims about the effectiveness of ENDS (10).
A study by Kennedy et al. published in 2017 (12), scanned the globe looking for countries that used policies to regulate E-CIGs/ENDS. It was focused on the specific regulatory mechanisms and regulatory domains. Overall, 68 countries in the 6 continents were identified that regulated E-CIGs at a national level using a range of regulatory mechanisms, such as new or amended laws, alerts, circulars, notifications, ordinances, statements. Domains of regulations included manufacturing, distribution, importation, sale (including where sales were allowed and minimum age of purchase), use of restriction including vape-free public places, advertising, promotion, health warning labelling, ingredients/flavors, safety/hygiene, reporting/notification, nicotine volume/concentration and child-safety packaging. Around a third of countries did not have specific regulations for E-CIGs and translated existing tobacco control regulations for these products. Several countries (n=25) banned E-CIGs use in enclosed public spaces and workplaces; also advertising and promotion was explicitly banned in 35 countries. There was also a wide variety of packaging and some heterogeneity about health warnings on packaging as well. Only few countries were applying a tax to E-CIGs (11).
In Europe, ENDS use is regulated by the EU Tobacco Products Directive (TPD) revised in 2016 which introduced rules for tobacco-related products across the 28 EU Member States. TPD both mandates and suggests a range of policy domains for regulating ENDS. The nine mandated provisions include reporting and notification, safety and quality, packaging and labelling and advertising/promotion/sponsorship; suggested provisions include regulations around importation and cross-border sales, application of taxes, vape-free laws and minimum age of purchase (12).
Regulatory mechanisms
As described in the study conducted by Kennedy et al., countries use a variety of regulatory approaches: (I) establish specific new laws, decrees, resolutions for regulating ENDS; (II) use existing legislation that can be applied if the classification of ENDS falls within the existing legal framework; (III) amend existing laws to address ENDS; (IV) combination of these measures.
More recently, in 2018 WHO provided an updated report on regulatory and market developments in ENDS and ENNDS (FCTC/COP/8/10). The report showed that ENDS were banned in 30 of the 195 WHO Member States globally (about 15%) and only about 65 Member States in which ENDS were not banned had regulations. In 29 countries ENDS were regulated as therapeutic products, dependent or independent of the nicotine level. In 20 of those countries they were regulated as therapeutic or consumer products depending on the level of nicotine. In 18 countries they were regulated as tobacco products, and in 31 as consumer products—in some cases a combination of these regulatory treatments (13).
ENDS use and smoking cessation
The impact of E-CIG on smoking cessation is still controversial. To date, behavioral interventions with or without pharmacological therapy remain the standard of care, obtaining the highest quality of evidence. Nicotine replacement therapy (NRT), bupropion hydrochloride sustained-release and varenicline are the only pharmacotherapies approved by Food and Drug Administration (FDA) for the treatment of tobacco dependence. The approval was based on more than 50 systematic reviews showing an improvement in smoking cessation rates from 10% (in control groups) to 17% with NRT, from 11% to 19% with bupropion, and from 12% to 28% in varenicline (14).
In a randomized trial, 886 patients were assigned to either NRT with products of their choice or second-generation E-CIG. Both arms were accompanied by behavioral support. The 1-year abstinence was the primary outcome and was reached by 9.9% of NRT users compared with 18.0% of E-CIG users (15). In contrast, several studies and meta-analyses question whether ENDS could be a valid smoking cessation method. A recent systematic review and meta-analysis, found that E-CIG users had a 28% lower probability of smoking cessation (OR 0.72). Authors included 38 studies in the systematic review. Among these studies, 20 had a control group and were included in the meta-analysis. Despite the heterogeneity of the studies in terms of design, study type, populations (all smokers vs. smokers motivated to stop smoking) and other characteristics, the sensitivity analysis confirmed the strength of the results (16). Similar results were obtained from a large Italian survey, which included 6,112 adults who tried to quit smoking using different methods. Smoking abstinence was reported among 9% of those using no aid; 8% of e-cigarette users; 15% of those using other methods. Interestingly, 6-months smoking cessation rates were lower in those who used E-CIG (8%) or did not use any method (9%), compared with those who used medications, or specific smoking cessation programs provided by accredited services (15%) (17). Similarly, in a prospective cohort US study, 1,284 adult smokers were followed. At baseline, the frequency of dual-users was 27.1%. One year later, only 9.2% of dual-users reached smoking cessation, while 53.5% continued to smoke ENDS and cigarettes and 37.4% only traditional cigarettes. Quitting smoking was reached by 18.9% of traditional cigarette-only users and 9.4% of ENDS users (adjusted OR 0.17) (18).
The early-age use of ENDS raises questions
ENDS exert a significant appeal on youth for several reasons. Firstly, ENDS have a wide choice of pleasing flavors and attractive designs; secondly, they are considered less harmful compared to traditional cigarettes; thirdly, they are used as socialization medium. However, the use of ENDS in young people is one of the most worrying aspects of their spread. A great percentage of young people use E-CIG as the first product containing nicotine. ENDS can therefore represent the gateway for the use of other nicotine products including usual cigarettes. Moreover, among the critical issues that deserve special attention, there are the acute and chronic nicotine-induced effects on the adolescent's brain that determine changes that could persist into adulthood. These aspects will be detailed later in the review.
According to the 2011–2018 National Youth Tobacco Survey, in US, from 2015 to 2017 there was an overall decline in the use of any tobacco product among middle and high school students. However, starting from 2017, this virtuous trend was interrupted, recording constantly increasing percentages of use for ENDS and traditional cigarettes. In particular, the use of E-CIG has clearly increased in high school students, from 1.5% in 2011 to 20.8% in 2018 (from 220,000 students to 3,050,000, respectively). The greatest increase was reported in 2017–2018, ranging from 11.7% to 20.8%. ENDS have become widespread even among middle school students. While in 2011 the percentage of users was 0.6%, in 2018 it increased to 4.9%, involving more than half a million students (19). Another US cross-sectional analysis showed that youth aged 12–17 years have a predilection for flavored tobacco products, with a percentage of use of 79.8% among current tobacco users. In contrast, only 28.6% of smokers aged ≥65 years use flavored products. Interestingly, 81% of young tobacco users (aged 12–17 at first use) stated that their first product was flavored (20).
In response to the concern upon the youth use of ENDS, FDA created the Youth Tobacco Prevention Plan, a series of actions aiming at stopping youth use of tobacco products, especially e-cigarettes, with special focus on three areas: preventing youth access to tobacco products, curbing marketing of tobacco products aiming at youth, educating teens and their families about the dangers of tobacco products (21).
ENDS and cancer: truths, uncertainties and perspectives
A critical issue is the presence of potentially toxic components in the vapor emitted by several devices. Some of these chemical components could increase the risk of developing cancer. Among the substances emitted by some E-CIGs, some carcinogens were found, including formaldehyde and acetaldehyde. These chemical constituents are produced by the exposure of the solvent to the high temperature. The solvent can contain propylene glycol and vegetable glycerin causing the release of carbonyl compounds when the heating element increases the temperature (22,23). To date, formaldehyde and acetaldehyde are classified by the International Agency for Research on Cancer (IARC) as a Group 1 and 2B carcinogens, respectively, based on appropriate evidence mainly for nasopharyngeal cancer, leukemia and upper digestive tract cancers. Formaldehyde acts through the formation of covalent bonds with DNA and proteins. Acetaldehyde reacts with DNA generating adducts, particularly in people consuming a high proportion of alcohol. The impact of acetaldehyde is especially enhanced by alcohol drinking and tobacco smoking with their known synergistic effect (24,25).
The extent of the problem is difficult to estimate because there are so many brands of E-CIGs and lots of e-liquids containing a wide choice of flavors. In a compositional analysis, a multitude of potential toxic compounds was detected across different devices. First of all, there was a great heterogeneity of the nicotine content, ranging from 0 to 37 mg/ml. Secondly, measurable quantities of formaldehyde, acetaldehyde and acrolein were found in 34.4%, 10.9% and 2.2% of e-liquids, reaching maximum concentrations of 24, 300 and 1.6 µg/mL, respectively. Rarely, measurable quantities of diethylene glycol, probably as a contaminant, benzene, toluene were detected. In addition, diacetyl, other aldehydes and ketones, metals, other tobacco specific nitrosamines and volatile organic compounds were present in several e-liquids in really different concentrations. Furthermore, the vaporization process can alter the concentrations of these components. When e-liquids were vaporized, indeed, the concentrations of several aldehydes and metals increased (22).
Using 10 different commercialized e-liquids and 3 control solutions, a quantitative analysis found that several second-generation ENDS delivered a variety of substances in different levels and composition depending on the device. Among the compounds released in 15 puffs of vapor, formaldehyde, acetaldehyde, acetone, butanol, benzaldehyde, crotonaldehyde, isovaleric aldehyde and m-methylbenzaldehyde were detected from at least one device. In this study, e-liquid containing propylene glycol had a significantly higher emission of carbonyls compared with vegetable glycerin (P<0.05). In addition, the high output voltage of the battery (4.8 V compared with 3.2 V) was associated with greater delivery of toxic substances (e.g., concentrations of formaldehyde, acetaldehyde and acetone from 4 to over 200 times higher), reaching levels quite similar to the usual cigarettes (23).
A cross-sectional study conducted in UK analysed expired air, urine and saliva samples from 109 current smokers (more than or equal to 5 cigarettes per day for at least 6 months) and 72 ex-smokers (NRT-users and E-CIG-users with no tobacco consumption for at least 6 months). Despite nicotine intake was quite similar across the groups, nicotine, cotinine and tobacco alkaloids (anabasine and anatabine) levels were lower in dual users (NRT-cigarettes) compared with cigarette smokers. Similarly, nicotine levels and alkaloids were lower in E-CIG consumers compared with usual cigarette smokers. Volatile organic compounds (in specific acrylamide, acrolein, acrylonitrile, vinyl chloride, ethylene oxide and 1,3-butadiene) and tobacco-specific nitrosamine (in specific 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone) levels were higher with traditional cigarette consumption, with similar values in dual users and cigarette-only users (24).
ENDS effects in other diseases
Recently, cases of patients who presented a pulmonary disease temporally related to E-CIG consumption were described in Illinois and Wisconsin. In this case series, patients were generally in good health, with ages ranging from 16 to 53 years (median age of 19 years), without major respiratory comorbidities, and had used E-CIG within the 90 days before presenting symptoms. Tetrahydrocannabinol, often in an oily solvent, was a constituent element in 80% of emissions, nicotine in 61% and both in 44%. Symptoms included dyspnea in 87%, cough in 83%, chest pain in 55%, nausea in 70%, vomiting in 66%, subjective fever in 81%, and leukocytosis in 87%, usually due to an increased number of neutrophils. CT scan commonly showed ground-glass areas, often associated with subpleural sparing. Pleural effusion, pneumomediastinum and pneumothorax were occasionally reported. Overall, close to one third of patients underwent intubation and assisted ventilation due to severe respiratory failure and one patient died (26). After the publication of this case series, other authors reported similar cases in other geographical areas. Six cases of possible lung injury related to E-CIG use have been detected in Utah. Signs and symptoms were similar to those reported in Illinois and Wisconsin, with neutrophilic leukocytosis, cough, dyspnea, nausea, vomiting and altered imaging. CT images were consistent with the pattern of lipoid pneumonia and correlated with the presence of lipid-laden macrophages isolated from bronchoalveolar lavage (27). Four main radiological patters related to electronic vapor exposure were identified. Imaging patterns included lipoid pneumonia, acute eosinophilic pneumonia, diffuse alveolar damage, organizing pneumonia, and other radiological findings (28). Day by day, the reports of cases of vaping-associated severe pulmonary disease have been exponentially increasing. As of September 20, 2019, more than 900 cases, of which 495 confirmed, have been reported across the United States (29).
Although the exact mechanism of vaping-associated severe pulmonary disease and the substances responsible for this pulmonary syndrome are not yet known, several in vitro and in vivo researches had already identified a lot of safety issues (30-32).
As previously reported, the high output voltage of the battery is responsible for a greater emission of toxic substances (23). In a randomized, single-blinded, ongoing trial, 25 smokers were randomly assigned to receive some puffs from an E-CIG containing propylene glycol and glycerol vaporized at 60 W or placebo. In this study, hypoxia in skin tissue, airway damage, as assessed by the increase in the marker for the integrity of lung epithelium (CC16) and increase in small airway resistance were observed in the smoking exposure group (30).
Using a lung epithelial cell line (CALU3 cells), some authors have shown a dose-dependent reduction of cell proliferation and viability with several flavors, propylene glycol, and vegetable glycerin, contained in e-liquids, after exposure to vapor or directly to e-liquids. Although all the flavors are involved in the reduction of cell proliferation and vitality, the intensity of this reduction is different among the various flavors (31). Another line of research concerns the role of ENDS in the immune system impairment, particularly affecting the ability of the mucosal immunity (32,33).
Collecting total RNA from nasal scrape biopsies and analyzing the expression of a specific panel of immune-related genes, a reduction in the expression of several genes associated with the immune response was observed in both traditional smokers and E-CIG smokers, but not in non-smokers. In this prospective study, 358 out of 597 genes were differently expressed in E-CIG smokers than non-smokers. These genes directly involved in immunological processes included ZBTB16, PIGR, PTGS2, FKBP5, and EGR1. The reduction of EGR1 expression leads to a decreased transcription of CD44, CSF1, CXCL2, BCL2L11, and FAS (32). In vivo comparing murine models exposed to air or E-CIG vapor, an association between E-CIG exposure and compromised pulmonary defenses against bacterial and viral infections was shown. In specific, mice exposed to E-CIG emission had significantly higher macrophage infiltration, lower IL2 levels, and higher concentration of thiobarbituric acid reactive substances compared with mice exposed to air. In addition, after intranasal inoculum of S. Pneumoniae, an impaired bacterial clearance associated with compromised phagocytic function was observed in E-CIG exposed mice. Similarly, exposing mice to a modified H1N1 influenza virus, higher viral titers in lungs, weight loss and mortality were observed in E-CIG exposed mice (33). The critical points on ENDS safety are not limited to pulmonary toxicity. Other significant issues concern the possible damage to cardiomyoblasts and the neuroplastic changes due to nicotine exposure during adolescence (34-37).
Considering the oxidative stress on myocardial cells caused by tobacco smoking, a group of researchers evaluated the free radical emissions from the E-CIG and the impact on cultured cardiomyoblasts compared with the effects from cigarette smoke exposure. Despite cell viability parameters worsened after exposure to high voltage vaping, most of the analyzed E-CIGs (80%) did not cause cardiomyoblast cytotoxicity. In contrast, exposure to tobacco smoke significantly decreased cell survival, producing apoptotic effects and necrosis with DNA damage and mitochondrial impairment probably due to oxidative stress (34).
Other aspects that deserve a special attention are the neuroplastic changes and the neuronal modifications under short or chronic exposure to nicotine, particularly in vulnerable people like adolescents. Investigating prefrontal attentional network function in young adults (age ranging from 20 to 25 years) with the use of functional magnetic resonance, some authors observed a significant reduction in the prefrontal attentional network activity among smokers compared with non-smokers. This finding was consistent with the available literature and correlated significantly with the duration of the smoking habit (35). A consistent alteration of cognitive functions has also been detected in animal models exposed to nicotine. In adolescent rats, a reduction in cognitive performance that was prolonged into adulthood was observed. This cognitive impairment was associated with a decrease in visuospatial attentional and an increase in impulsive actions. These long-term nicotine-induced effects are mainly pronounced in cases where exposure occurs during adolescence, when the development of the nervous system can receive permanent changes involving the dopamine signaling at the medial prefrontal cortical areas (36). In addition, using mice chronically exposed to nicotine-containing E-CIG, reductions of dopamine in the striatum, GABA in the frontal cortex and glutamine in either frontal cortex or striatum were observed (37).
Finally, a recent report by the US Centers for Disease Control and Prevention about the association between vitamin E acetate and lung injury in ENDS smokers suggested a potential role for vitamin E acetate in the e-cigarette, or vaping, product use associated lung injury outbreak (38).
However, a precise and homogeneous estimate of the long-term effects of ENDS is difficult to make for the relatively short time of marketing and the continuous replacement of models.
Conclusions
To date, no ENDS received FDA approval for smoking cessation and ENDS use should not be admitted to as being the lesser of two evils. As previously detailed, indeed, there is no clear evidence on the efficacy of ENDS use to stop smoking. Given the extremely varied types of ENDS and the heterogeneous concentrations of toxic substances emitted by specific E-CIGs, an overall risk assessment is particularly difficult. Several efforts should still be made to fully understand the acute and long-term effects of ENDS use, with attention to young users.
The WHO represents an authoritative voice in this field; providing updated information and indications that should guide the choices of states, communities and individuals.
Acknowledgments
We thank Anna Roviello for the help given in the creation and development of the figure.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Alfredo Tartarone) for the Series “Improving Outcomes in Lung Cancer Through Early Diagnosis and Smoking Cessation” published in Journal of Thoracic Disease. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See:
References
- World Health Organization. WHO Grobal Report: Mortality Attributable to Tobacco. Geneva, Switzerland: World Health Organization; 2012. Available online: https://www.who.int/tobacco/publications/surveillance/rep_mortality_attributable/en/
- World Health Organization. WHO Report on the Global Tobacco Epidemic, 2019. Geneva: World Health Organization; 2019. Licence: CC BY-NC-SA 3.0 IGO. Available online: https://apps.who.int/iris/bitstream/handle/10665/325968/WHO-NMH-PND-2019.5-eng.pdf?ua=1
- Unger M, Unger DW. E-cigarettes/electronic nicotine delivery systems: a word of caution on health and new product development. J Thorac Dis 2018;10:S2588-92. [Crossref] [PubMed]
- Williams M, Talbot P. Design Features in Multiple Generations of Electronic Cigarette Atomizers. Int J Environ Res Public Health 2019;16:2904. [Crossref] [PubMed]
- Global E-cigarettes market: Global industry trends, share, size, growth, opportunity and forecast 2019-2024. Available online: researchandmarkets.com
- Electronic Cigarette Market by Product Type, Flavor and Distribution Channel - Global opportunity Analysis and Industry Forecast, 2017-2023. Available online: https://www.researchandmarkets.com/research/pjkd84/globalelectronic?w=5
- Based on Euromonitor’s 2015 Data. Available online: https://www.euromonitor.com/smokeless-tobacco-and-vapour-products
- 2018 global progress report on implementation of the WHO. Available online: www.who.int/fctc/reporting/summary_analysis/
- Electronic Nicotine Delivery System and Electronic. Report by WHO FCTC/COP/6/10. Available online: www.who.int/fctc/publications
- Electronic Nicotine Delivery System and Electronic Non-nicotine Delivery Systems (ENDS/ENNDS). Report by WHO FCTC/COP/7/11. August 2016. Available online: https://www.who.int/tobacco/industry/product_regulation/en/
- Conference of the Parties to the WHO Framework Convention on Tobacco Control. Progress report on regulatory and market developments on electronic nicotine delivery systems (ENDS) and electronic non-nicotine delivery systems (ENNDS) 2018. Available online: https://www.who.int/fctc/cop/sessions/cop8/FCTC_COP_8_10-EN.pdf
- Kennedy RD, Awopegba A, De Leon E, et al. Global approaches to regulating electronic cigarettes. Tob Control 2017;26:440-5. [Crossref] [PubMed]
- Directive 2014/40/EU of the European Parliament and of the member states concerning the manufacture, presentation and sale of tobacco and related products and repealing directive 2001/37/EC. Available online: https://ec.europa.eu/health/sites/health/files/tobacco/docs/dir_201440_en.pdf
- Patnode CP, Henderson JT, Thompson JH, et al. Behavioral Counseling and Pharmacotherapy Interventions for Tobacco Cessation in Adults, Including Pregnant Women: A Review of Reviews for the U.S. Preventive Services Task Force. Evidence Synthesis. Rockville, MD: Agency for Healthcare Research and Quality; 2015 Sep. Report No.: 14-05200-EF-1.
- Hajek P, Phillips-Waller A, Przulj D, et al. A Randomized Trial of E-Cigarettes versus Nicotine-Replacement Therapy. N Engl J Med 2019;380:629-37. [Crossref] [PubMed]
- Kalkhoran S, Glantz SA. E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis. Lancet Respir Med 2016;4:116-28. [Crossref] [PubMed]
- Gorini G, Ferrante G, Quarchioni E, et al. Electronic cigarette use as an aid to quit smoking in the representative Italian population PASSI survey. Prev Med 2017;102:1-5. [Crossref] [PubMed]
- Weaver SR, Huang J, Pechacek TF, et al. Are electronic nicotine delivery systems helping cigarette smokers quit? Evidence from a prospective cohort study of U.S. adult smokers, 2015-2016. PLoS One 2018;13:e0198047. [Crossref] [PubMed]
- Cullen KA, Ambrose BK, Gentzke AS, et al. Notes from the Field: Increase in use of electronic cigarettes and any tobacco product among middle and high school students — United States, 2011-2018. MMWR Morb Mortal Wkly Rep 2018;67:1276-7. [Crossref] [PubMed]
- Villanti AC, Johnson AL, Ambrose BK, et al. Flavored Tobacco Product Use in Youth and Adults: Findings From the First Wave of the PATH Study (2013-2014). Am J Prev Med 2017;53:139-51. [Crossref] [PubMed]
- FDA's Youth Tobacco Prevention Plan 2019. Available online: https://www.fda.gov/tobacco-products/youth-and-tobacco/fdas-youth-tobacco-prevention-plan
- Visser W, Geraets L, Klerx W et al. The health risks of using e-cigarettes. Bilthoven The Netherlands: National Institute for Public Health and the Environment; 2015. Available online: http://www.rivm.nl/bibliotheek/rapporten/2015-0144.pdf
- Kosmider L, Sobczak A, Fik M, et al. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res 2014;16:1319-26. [Crossref] [PubMed]
- International Agency for Research on Cancer (IARC). Agents Classified by the IARC Monographs, Volumes 1-123 (last update 16 July 2019). Available online: https://monographs.iarc.fr/wp-content/uploads/2018/09/ClassificationsAlphaOrder.pdf
- Ward EM, Schulte PA, Straif K, et al. Research recommendations for selected IARC-classified agents. Environ Health Perspect 2010;118:1355-62. [Crossref] [PubMed]
- Layden JE, Ghinai I, Pray I, et al. Pulmonary Illness Related to E-Cigarette Use in Illinois and Wisconsin - Preliminary Report. N Engl J Med 2020;382:903-16. [Crossref] [PubMed]
- Maddock SD, Cirulis MM, Callahan SJ, et al. Pulmonary Lipid-Laden Macrophages and Vaping. N Engl J Med 2019;381:1488-9. [Crossref] [PubMed]
- Henry TS, Kanne JP, Kligerman SJ. Imaging of Vaping-Associated Lung Disease. N Engl J Med 2019;381:1486-7. [Crossref] [PubMed]
- Hswen Y, Brownstein JS. Real-Time Digital Surveillance of Vaping-Induced Pulmonary Disease. N Engl J Med 2019;381:1778-80. [Crossref] [PubMed]
- Chaumont M, Bernard A, Pochet S, et al. High-Wattage E-Cigarettes Induce Tissue Hypoxia and Lower Airway Injury: A Randomized Clinical Trial. Am J Respir Crit Care Med 2018;198:123-6. [Crossref] [PubMed]
- Rowell TR, Reeber SL, Lee SL, et al. Flavored e-cigarette liquids reduce proliferation and viability in the CALU3 airway epithelial cell line. Am J Physiol Lung Cell Mol Physiol 2017;313:L52-66. [Crossref] [PubMed]
- Martin EM, Clapp PW, Rebuli ME, et al. E-cigarette use results in suppression of immune and inflammatory-response genes in nasal epithelial cells similar to cigarette smoke. Am J Physiol Lung Cell Mol Physiol 2016;311:L135-44. [Crossref] [PubMed]
- Sussan TE, Gajghate S, Thimmulappa RK, et al. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One 2015;10:e0116861. [Crossref] [PubMed]
- Farsalinos KE, Romagna G, Allifranchini E, et al. Comparison of the cytotoxic potential of cigarette smoke and electronic cigarette vapour extract on cultured myocardial cells. Int J Environ Res Public Health 2013;10:5146-62. [Crossref] [PubMed]
- Musso F, Bettermann F, Vucurevic G, et al. Smoking impacts on prefrontal attentional network function in young adult brains. Psychopharmacology (Berl) 2007;191:159-69. [Crossref] [PubMed]
- Counotte DS, Spijker S, Van de Burgwal LH, et al. Long-lasting cognitive deficits resulting from adolescent nicotine exposure in rats. Neuropsychopharmacology 2009;34:299-306. [Crossref] [PubMed]
- Alasmari F, Crotty Alexander LE, Hammad AM, et al. Effects of Chronic Inhalation of Electronic Cigarette Vapor Containing Nicotine on Neurotransmitters in the Frontal Cortex and Striatum of C57BL/6 Mice. Front Pharmacol 2019;10:885. [Crossref] [PubMed]
- Taylor J, Wiens T, Peterson J, et al. Characteristics of E-cigarette, or Vaping, Products Used by Patients with Associated Lung Injury and Products Seized by Law Enforcement - Minnesota, 2018 and 2019. MMWR Morb Mortal Wkly Rep 2019;68:1096-100. [Crossref] [PubMed]


