Radiation protection in the cardiac catheterization laboratory
Introduction
Occupational radiation exposure is a major concern for cardiac catheterization laboratory workers. Radiation has no minimum safety threshold and its adverse effects occur in a linear, dose-dependent risk (1). Ionizing radiation’s harmful effects on human tissues have been recognized to either be deterministic or stochastic. Deterministic radiation injuries refer to cell death when the exposure exceeds a certain threshold. Examples are cataracts, skin erythema and desquamation, and sterility (2). Stochastic effects refer to injuries that occur in proportion to cumulative radiation dose over time. They have a long latency period and there is no threshold dose below which genetic damage will not occur. These effects are typically cancers of the skin, thyroid gland, nervous system and gastrointestinal tract (3).
Occupational radiation exposure in the cardiac catheterization laboratory
Interventional cardiologists are at risk from radiation injury given their chronic radiation exposure in cardiac catheterization laboratories. Compared to clinical cardiologists who work outside the cardiac catheterization laboratory, they develop somatic DNA damage and chromosomal abnormalities at a higher frequency when measured using micronuclei assay in peripheral lymphocytes (4). There also have been reported cases of brain tumors among physicians performing interventional procedures occurring disproportionately on the left side. It is known that radiation exposure occurs two times more on the left side of the head compared to the right (5). Furthermore, in the cataracts attributed to radiation in the cath lab study (IC-CATARACT), workers in the cardiac catherization laboratory have been found to have a higher incidence of cataracts. Occupational exposure and age over 60 were also found to be predictors of sublcinical lens changes (6). Compared to those without fluoroscopy exposure, interventional cardiologists have as much as three times increased rate posterior subcapsular lens opacities (7).
Similarly, compared to those who work in an operating room setting, anesthesiologists working in the cardiac catheterization laboratory are exposed to significantly more radiation (8). A personal dose meter placed on an anesthesia machine has been shown to receive 15 times the radiation compared to the dosimeter worn by a scrub nurse (9). The position of the anesthesiologists on the left side of the patient where it is shown to receive more scatter radiation suggests that they may be exposed to more radiation dose than interventional cardiologists who are positioned on the right side of the patient (9,10). These findings may be due to ineffective shielding as anesthesiologists frequently need to have direct patient contact during the procedure. This is compounded by their relatively fixed position during the procedure and inability to maintain a safe distance from the radiation source due to space limitation (10). Scrub nurses, on the other hand, are mobile and their distance from the radiation source varies. Their position behind leaded shields or behind leaded operators gives them also additional protection (11). Unlike the operator, anesthesiologists and cardiac catheterization laboratory staff have the disadvantage of not having control over the duration of the procedure or the amount of radiation utilized during the case.
A special population of concern in the cardiac catheterization laboratory is pregnant women. Female cardiology fellows and interventionalists make up around 20% and 8% of cardiology fellows and interventional cardiology physicians, respectively (12,13). Female nurses, anesthesiologists, and other staff are also at risk for radiation exposure during pregnancy. In utero radiation exposure has deleterious effects in the fetus. Organ malformation occurs at a threshold dose of 250 mGy; intrauterine growth retardation, 200 mGy; delayed mental development, 100 mGy. No threshold, however, was observed for childhood cancers (14). Compared to the general population exposed to background radiation, there is no difference in fetal outcomes in women who were exposed to a cumulative radiation dose of <50 mGy during pregnancy (15). For pregnant women, the National Radiation Protection and Measurement has set an allowable occupational radiation dose to 0.5 mSv per month or a total of 5 mSv during the span of the pregnancy (16).
Radiation exposure in different fluoroscopy-guided procedures
Different interventional procedures have substantial variability in radiation dose exposure. Among the three, interventional radiology procedures have been found to have the highest radiation dose, followed by interventional cardiology, while electrophysiology procedures have the least exposure (17). A possible explanation for this is that interventional radiology procedures have longer fluoroscopy times and utilize higher dose per frame rate especially when working on pelvic areas and endovascular systems. As for electrophysiology procedures, 3D-electroanatomic mapping and multimodal imaging have enabled operators to reduce fluoroscopy use resulting in less radiation exposure (18).
The procedures with the highest radiation exposure are structural or valvular cardiac procedures followed by peripheral vascular procedures (17). Of the structural cardiac procedures, fluoroscopy time and radiation dose are greatest for percutaneous transcatheter aortic valve implantation, transcatheter mitral-valve approximation with MitraClip, and left atrial appendage closures (19). A possible reason for this is that these were relatively new procedures in their institution at the time of study. Hence, there are longer case times and higher radiation exposures as operators are still getting experience with the procedures (19). Studies are mixed when radiation dose in structural cardiac procedures were obtained and compared to percutaneous intervention; they were either found to be significantly greater or similar (19,20). On the other end of the spectrum, pacemaker insertion and electrophysiology ablation procedures were associated with the least radiation dose (17).
Radiation protection
Radiation protection consists of two components - passive and active processes (Table 1). The passive component consists of the protective equipment in the laboratory, while the active component is based on the use of these equipments. Active protection strategies include routine and appropriate use of lead apparel, proper training of the staff on radiation exposure, routine radiation dose monitoring, and using techniques in reducing radiation use to the patient and operator (21,22).

Full table
Shielding
Shielding in the cardiac catheterization laboratory can be categorized into three types: architectural, equipment-mounted, and personal protective devices (Figure 1). Architectural shielding is built into the cardiac catheterization laboratory structure. This also includes rolling and stationary leaded transparent plastic shields that protect nursing staff and anesthesiologists (23).
Equipment-mounted shielding consists of ceiling-suspended shields, table-suspended drapes, and disposable protective patient drapes. Ceiling-suspended shields are typically made of transparent leaded plastic that are readily adjustable during the procedure. Precise positioning of this is the key in significantly reducing operator exposure. There is a gap in protection created by the patient contour cutout and to minimize this, the upper body shield should be located far from the scatter source and near the operator. For example, in femoral artery access sites, it should be positioned just cephalad to the groin and as close as possible to the patient surface. Throughout the procedure, frequent repositioning of the upper body shield should be kept in mind as the table is moved to maintain effective protection (24).
The under-table X-ray tube gives off significant scatter radiation that is not usually covered by lead aprons. Table-suspended drapes or lead curtains between the X-ray tube and the operator provide protection from it. In the extended protective shield under table to reduce operator radiation dose in percutaneous coronary procedures (EXTRA-RAD) study, the use of under-table anti-radiation shields (drapes or curtains) resulted in lower radiation dose exposure at the pelvic and thorax level of the operators (25). In another study, installation of protective curtains has been shown to lead to a radiation dose reduction of as much as 64% (26).
Disposable radioabsorbent drapes that consist of bismuth, barium or tungsten-antimony have been shown to be effective in reducing radiation doses (27). These are placed on the patient and have been demonstrated to reduce attenuate scatter radiation twelve-fold for the eyes, twenty five-fold for the thyroid, and twenty-nine-fold for the hands (28). However, a study showed that although use of a pelvic lead shield for trans-radial interventions decreases radiation threefold for the operator, it doubled the exposure to the patient. A possible explanation was that the lead apron protected the operator from scatter radiation, but most of the radiation to the patient is related to the ones that the patient absorbs and does not leave the body. It has been argued that the cumulative dose of radiation to the operator significantly increases lifetime risk of cancer, while the small incremental increase in radiation translates to a clinically negligible amount for the patient (29).
A transradial radiation protection board has been shown to reduce radiation operator dose during radial approach procedures. It consists of a 20-cm vertical shield inserted in a wide base that provides support for the patient’s wrist when obtaining radial access (30). Rad Board® radial arm board is a similar device, but does not have this vertical shield. Instead, it has a built-in radiation scatter protection to its arm support. In an independent survey featured in its website, it was shown that it reduces up to 44% and 25% radiation dose at waist height and at neck height, respectively (31). A subsequent randomized controlled trial, however, showed that utilizing it is significantly associated with more radiation exposure to the operator (32). In this study, setting up the Rad Board precluded the use of a 6-inch vertical shield typically inserted on the side of the patient between the below-table and the ceiling-mounted shield in the study.
Personal protective equipment
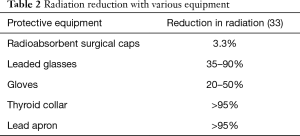
Personal protective equipment includes caps, gloves, eyewear, wearable aprons, and thyroid collar (Table 2). Although risk of malignancy is low, reports of left-sided brain cancer in interventional cardiologists and electrophysiologists may suggest a causal relation to occupational radiation exposure and has been an area of concern (5). Compared to ceiling-mounted lead shields, lead caps that cover the sides and lower parts of the face have been shown decrease head radiation exposure (34). However, a lead cap approximately weighs 1,140 grams which may further contribute to operators’ orthopedic injuries (35). There are now available lead-free alternatives like surgical caps consisting bismuth and barium that weigh only 53 g. These were originally accepted as effective in reducing radiation dose (36). However, a more recent study showed that radiation scatter predominantly comes from below the physician’s head and radioabsorbent surgical caps do not cover this area. It has been shown to decrease radiation dose to the brain by only 3.3%—an almost negligible amount (24). When it comes to gloves, exposure to radiation of the hands does not pose a significant health risk. The best way to protect the hands is to keep them away from the direct radiation beam. There are radiation attenuating gloves available but their large size limit their use especially since cardiac catheterization laboratory procedures require manual dexterity (37).
Full table
Radiation-induced cataract or loss of transparency of the lens of the eye is one of the well-known risks in the cardiac catheterization laboratory. For acute exposures, the threshold for cataract induction is between 0.5 and 2 Gy, while it is 5–6 Gy for protracted exposures (38). The use of leaded glasses has been shown to reduce eye radiation by 35– >90% and when the sides of the eyes are protected like in a lead acrylic face mask, radiation exposure can be reduced by as much as 97% (33,39). The thyroid gland is a radiosensitive organ and thyroid cancer is a known consequence of radiation exposure. Although the cancer risk from radiation decreases significantly when exposure occurs after 20 years of age, a thyroid shield should always be worn during procedures (40). Care must be taken when wearing it and make sure there are no gaps between the thyroid shield and the lead apron.
The main radiation protection tool for the cardiac catheterization laboratory workers is the apron. It is important in protecting the bone marrow and reproductive organs. A full body apron, however, weighs around 7 kg and can cause back problems (3). An alternative is a two-piece wraparound apron consisting of a skirt and a vest. This type of garment is lighter and it shifts some of the weight to the hips, alleviating the load from the shoulders and spine (37). The fit of the apron is important for ergonomic and safety purposes. Large gaps, for example, under the arms can expose breast tissue to radiation and put female staff at risk for breast cancer (41). There are now lead composite or lead-free aprons that have 20–40% reduced weight (42). From around 7 kg, these newer generation protective aprons weight around 4 kg. This reduces the risk of musculoskeletal injury for the staff stemming from the lead apron’s weight. To completely remove lead weight from the operator, there is a Zerogravity system available which is a ceiling-suspended personal radiation protection system (43). It consists of a curved lead head shield and a lead apron with arm flaps to the elbows (Figure 1). It has been shown to reduce radiation exposure 16–78 fold compared to conventional aprons (44).
Pregnant women in the cardiac catheterization laboratory
There are multiple strategies that can be employed for radiation exposure that are specific to pregnant women. These include making sure personal protective equipment measures at least 0.5 mm lead equivalent, doubling the thickness of lead aprons or wearing specific maternity lead aprons, and having an additional dosimeter at waist level under the lead apron to monitor fetal radiation exposure (45). It is important to note that federal laws prohibit discriminating against pregnant women in the workplace. With proper protection, they should not be prevented from working in the cardiac catheterization laboratory (46).
Techniques to reduce radiation exposure
During the procedure itself, there are several techniques that can be utilized to decrease radiation dose. For example, limiting fluoroscopy time to only when the operator is looking at the monitor. Instead of using more fluoroscopy to study the coronary arteries, review last image hold or use fluoroscopy loop for dynamic processes. Similarly, use of high dose modes such as high contrast mode, override mode or boost mode should be minimized. Adjusting fluoroscopy frame rate can also contribute to reducing radiation exposure. Although it can lower image quality, lower frame rates are usually adequate in many instances. Frame rate is typically set at 15 frames-per-second and decreasing it to 7.5 frames-per-second has been shown to result in significant radiation dose reduction (47). It is similarly paramount to utilize all available radiation dose reduction technologies. These include virtual collimation, last image hold, and storage of fluoroscopy. Other radiation sparing features are low pulse-rate fluoroscopy options, low dose-per-frame, low frame rate options for image acquisition, spectral beam filtration and higher X-ray beam energy (48). Relatively new image noise reduction technology has half the amount of radiation dose compared to traditional fluoroscopy (49). Adjusting technical settings of X-ray equipment and implementing dose reduction protocols has been shown to decrease radiation exposure by 48% (50).
Optimal table positioning can reduce radiation dose to the patient. The patient is ideally placed as close as possible to the image receptor, and further away from the X-ray source. A higher table setting decreases patient skin dose. Steep angulation should also be avoided as this increases scatter radiation, radiation dose rate, and operator exposure (51). Ensuring tight collimator blade placement results in better image quality and decreases scatter radiation. Similar results can be gained from the utilization of semi-transparent or wedge filters (48).
During the procedure, maintaining distance from the X-ray beam and positioning themselves in a low scatter area reduces radiation exposure for the operator and staff. Because of automatic dose controls that increase X-ray tube output with patient thickness, it is important to keep patient's non-target anatomy and operator hands away from the field of view or primary X-ray beam (52). Choice of access site also affects radiation exposure. Radiation exposure in transradial approach decreases with operator experience (53-56). However, the use of radial access sites has been shown to have a small, but significant increase in radiation dose exposure as opposed to femoral access sites (57).
Future directions
Given the harmful effects of radiation, several technological advancements have been developed that will help reduce radiation exposure to the operator, patient, and staff in the cardiac catheterization laboratory. Intravascular ultrasound (IVUS) provides detailed coronary arterial wall architecture and lesion morphology. In complex interventions, it helps decrease radiation dose exposure as it can give a more detailed view of the arterial lumen compared to fluoroscopy (58). Robotic remote-control angioplasty where interventional cardiologists work from a shielded workstation away from the radiation source have also been shown to reduce radiation dose by as much as 96% (59). Real-time radiation dose monitoring (Figure 2) has been shown to alter operator behavior during the procedure resulting in reduction in the peak skin and total radiation dose of the patient (60). More widespread use of this technology will be helpful in decreasing radiation exposure. Advocates have proposed including radiation dose reports in all interventional cardiology trials to emphasize its importance especially in the development of new imaging equipment (61). Further development in this area may dramatically decrease occupational health hazards for interventional cardiologists and cardiac catheterization laboratory staff.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Ion S. Jovin) for the series “Interventional Cardiology” published in Journal of Thoracic Disease. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: The series “Interventional Cardiology” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Valentin J. The 2007 recommendations of the international commission on radiological protection. ICRP publication 103. Ann iCRP 2007;37:1-332. [PubMed]
- Karatasakis A, Danek BA, Brilakis E. Radiation Protection. 2018:199-216.
- Smilowitz NR, Balter S, Weisz G. Occupational hazards of interventional cardiology. Cardiovasc Revasc Med 2013;14:223-8. [Crossref] [PubMed]
- Andreassi MG, Cioppa A, Botto N, et al. Somatic DNA damage in interventional cardiologists: a case-control study. FASEB J 2005;19:998-9. [Crossref] [PubMed]
- Roguin A, Goldstein J, Bar O, et al. Brain and neck tumors among physicians performing interventional procedures. Am J Cardiol 2013;111:1368-72. [Crossref] [PubMed]
- Karatasakis A, Brilakis HS, Danek BA, et al. Radiation-associated lens changes in the cardiac catheterization laboratory: Results from the IC-CATARACT (CATaracts Attributed to RAdiation in the CaTh lab) study. Catheter Cardiovasc Interv 2018;91:647-54. [Crossref] [PubMed]
- Duran A, Vano E, Kleinman NK, et al. Retrospective evaluation of lens injuries and dose: relid study. J Am Coll Cardiol 2011;57:E1951. [Crossref]
- Henderson KH, Lu JK, Strauss KJ, et al. Radiation exposure of anesthesiologists. J Clin Anesth 1994;6:37-41. [Crossref] [PubMed]
- Mohapatra A, Greenberg RK, Mastracci TM, et al. Radiation exposure to operating room personnel and patients during endovascular procedures. J Vasc Surg 2013;58:702-9. [Crossref] [PubMed]
- Dagal A. Radiation safety for anesthesiologists. Curr Opin Anaesthesiol 2011;24:445-50. [Crossref] [PubMed]
- Madder RD, LaCombe A, VanOosterhout S, et al. Radiation exposure among scrub technologists and nurse circulators during cardiac catheterization: the impact of accessory lead shields. JACC Cardiovasc Interv 2018;11:206-12. [Crossref] [PubMed]
- Colleges AoAM. ACGME Residents and Fellows by Sex and Specialty. 2017.
- Colleges AoAM. Active Physicians by Sex and Specialty. 2017.
- McCollough CH, Schueler BA, Atwell TD, et al. Radiation exposure and pregnancy: when should we be concerned? Radiographics 2007;27:909-17. [Crossref] [PubMed]
- Brent RL. The effect of embryonic and fetal exposure to X-ray, microwaves, and ultrasound: counseling the pregnant and nonpregnant patient about these risks. Semin Oncol 1989;16:347-68. [PubMed]
- Meinhold C, Abraharnson S, Adelstein S. Report No. 116—Limitation of Exposure to Ionizing Radiation (Supersedes NCRP Report No. 91). Bethesda, MD: National Council on Radiation Protection & Measurements 1993.
- Sciahbasi A, Ferrante G, Fischetti D, et al. Radiation dose among different cardiac and vascular invasive procedures: the RODEO study. Int J Cardiol 2017;240:92-6. [Crossref] [PubMed]
- Gaita F, Guerra PG, Battaglia A, et al. The dream of near-zero X-rays ablation comes true. Eur Heart J 2016;37:2749-55. [Crossref] [PubMed]
- Boland JE, Wang LW, Love BJ, et al. Radiation dose during percutaneous treatment of structural heart disease. Heart Lung Circ 2014;23:1075-83. [Crossref] [PubMed]
- Drury-Smith M, Maher A, Douglas-Hill C, et al. 25 TAVI operator radiation dose compared to PCI and ICD operators: do we need additional radiation protection for trans-catheter structural heart interventions. Heart 2011;97:A19-A.
- Bartal G, Vano E, Paulo G, et al. Management of patient and staff radiation dose in interventional radiology: current concepts. Cardiovasc Intervent Radiol 2014;37:289-98. [Crossref] [PubMed]
- Kim C, Vasaiwala S, Haque F, et al. Radiation safety among cardiology fellows. Am J Cardiol 2010;106:125-8. [Crossref] [PubMed]
- Lanzer P. Textbook of Catheter-Based Cardiovascular Interventions: A Knowledge-Based Approach. Springer, 2018.
- Fetterly KA, Magnuson DJ, Tannahill GM, et al. Effective use of radiation shields to minimize operator dose during invasive cardiology procedures. JACC 2011;4:1133-9. [PubMed]
- Sciahbasi A, Sarandrea A, Rigattieri S, et al. Extended Protective Shield Under Table to Reduce Operator Radiation Dose in Percutaneous Coronary Procedures: The EXTRA-RAD Study. Circulation 2019;12:e007586. [PubMed]
- Shortt CP, Al-Hashimi H, Malone L, et al. Staff radiation doses to the lower extremities in interventional radiology. Cardiovasc Intervent Radiol 2007;30:1206-9. [Crossref] [PubMed]
- Ertel A, Nadelson J, Shroff AR, et al. Radiation Dose Reduction during Radial Cardiac Catheterization: Evaluation of a Dedicated Radial Angiography Absorption Shielding Drape. ISRN Cardiol 2012;2012:769167.
- King JN, Champlin AM, Kelsey CA, et al. Using a sterile disposable protective surgical drape for reduction of radiation exposure to interventionalists. AJR Am J Roentgenol 2002;178:153-7. [Crossref] [PubMed]
- Musallam A, Volis I, Dadaev S, et al. A randomized study comparing the use of a pelvic lead shield during trans-radial interventions: Threefold decrease in radiation to the operator but double exposure to the patient. Catheter Cardiovasc Interv 2015;85:1164-70. [Crossref] [PubMed]
- Behan M, Haworth P, Colley P, et al. Decreasing operators' radiation exposure during coronary procedures: the transradial radiation protection board. Catheter Cardiovasc Interv 2010;76:79-84. [Crossref] [PubMed]
- Rad Board® Radial Arm Board. Available online: https://www.merit.com/cardiac-intervention/access/radial-approach-accessories/rad-board-radial-arm-board/#toggle-id-1. Accessed August 31 2019.
- Suryadevara R, Brown ED, Green SM, et al. A randomized controlled trial to assess operator radiation exposure from cardiac catheterization procedures using RAD BOARD® with standard pelvic shielding versus standard pelvic shielding alone. Catheter Cardiovasc Interv 2020;95:83-8. [Crossref] [PubMed]
- Kim KP, Miller DL. Minimising radiation exposure to physicians performing fluoroscopically guided cardiac catheterisation procedures: a review. Radiation Protection Dosimetry 2009;133:227-33. [Crossref] [PubMed]
- Kuon E, Birkel J, Schmitt M, et al. Radiation exposure benefit of a lead cap in invasive cardiology. Heart 2003;89:1205-10. [Crossref] [PubMed]
- Karadag B, Ikitimur B, Durmaz E, et al. Effectiveness of a lead cap in radiation protection of the head in the cardiac catheterisation laboratory. EuroIntervention 2013;9:754-6. [Crossref] [PubMed]
- Alazzoni A, Gordon CL, Syed J, et al. Randomized controlled trial of radiation protection with a patient lead shield and a novel, nonlead surgical cap for operators performing coronary angiography or intervention. Circulation 2015;8:e002384. [PubMed]
- Badawy MK, Deb P, Chan R, et al. A review of radiation protection solutions for the staff in the cardiac catheterisation laboratory. Heart Lung Circ 2016;25:961-7. [Crossref] [PubMed]
- Vanhavere F, Carinou E, Domienik J, et al. Measurements of eye lens doses in interventional radiology and cardiology: final results of the ORAMED project. Radiation Measurements 2011;46:1243-7. [Crossref]
- Marshall NW, Faulkner K, Clarke P. An investigation into the effect of protective devices on the dose to radiosensitive organs in the head and neck. Br J Radiol 1992;65:799-802. [Crossref] [PubMed]
- Ron E, Lubin JH, Shore RE, et al. Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res 1995;141:259-77. [Crossref] [PubMed]
- Baiter S, Rosenstein M, Miller DL, et al. Patient radiation dose audits for fluoroscopically guided interventional procedures. Med Phys 2011;38:1611-8. [Crossref] [PubMed]
- Papadopoulos N, Papaefstathiou C, Kaplanis P, et al., editors. Comparison of lead-free and conventional X-ray aprons for diagnostic radiology. World Congress on Medical Physics and Biomedical Engineering, September 7-12, 2009, Munich, Germany; 2009: Springer.
- Savage C, Seale TM IV, Shaw CJ, et al. Evaluation of a suspended personal radiation protection system vs. conventional apron and shields in clinical interventional procedures. Open J Radiol 2013;3:143. [Crossref]
- Marichal DA, Anwar T, Kirsch D, et al. Comparison of a suspended radiation protection system versus standard lead apron for radiation exposure of a simulated interventionalist. J Vasc Interv Radiol 2011;22:437-42. [Crossref] [PubMed]
- Best PJ, Skelding KA, Mehran R, et al. SCAI consensus document on occupational radiation exposure to the pregnant cardiologist and technical personnel. Heart Lung Circ 2011;20:83-90. [Crossref] [PubMed]
- Hood J. The pregnant health care worker—an evidence-based approach to job assignment and reassignment. Aaohn J 2008;56:329-33. [Crossref] [PubMed]
- Agarwal S, Parashar A, Ellis SG, et al. Measures to reduce radiation in a modern cardiac catheterization laboratory. Circulation 2014;7:447-55. [PubMed]
- Durán A, Hian SK, Miller DL, et al. Recommendations for occupational radiation protection in interventional cardiology. Catheter Cardiovasc Interv 2013;82:29-42. [Crossref] [PubMed]
- Gunja A, Pandey Y, Xie H, et al. Image noise reduction technology reduces radiation in a radial-first cardiac catheterization laboratory. Cardiovasc Revasc Med 2017;18:197-201. [Crossref] [PubMed]
- Wassef AW, Hiebert B, Ravandi A, et al. Radiation dose reduction in the cardiac catheterization laboratory utilizing a novel protocol. JACC 2014;7:550-7. [PubMed]
- Christopoulos G, Makke L, Christakopoulos G, et al. Optimizing radiation safety in the cardiac catheterization laboratory: a practical approach. Catheter Cardiovasc Interv 2016;87:291-301. [Crossref] [PubMed]
- Chambers JC, Zhao J, Terracciano CM, et al. Genetic variation in SCN10A influences cardiac conduction. Nat Genetics 2010;42:149. [Crossref] [PubMed]
- Ross J, Vidovich MI. Relative importance of attribute preferences for radial vs. femoral arterial access: A crowdsourcing study of healthy online-recruited volunteers. Catheter Cardiovasc Interv 2019;93:1237-43. [Crossref] [PubMed]
- Voudris KV, Habibi M, Karyofillis P, et al. Radiation Exposures Associated With Radial and Femoral Coronary Interventions. Curr Treat Options Cardiovasc Med 2016;18:73. [Crossref] [PubMed]
- Vidovich MI, Khan AA, Xie H, et al. Radiation safety and vascular access: attitudes among cardiologists worldwide. Cardiovasc Revasc Med 2015;16:109-15. [Crossref] [PubMed]
- Helfrich CD, Tsai TT, Rao SV, et al. Perceptions of advantages and barriers to radial-access percutaneous coronary intervention in VA cardiac catheterization laboratories. Cardiovasc Revasc Med 2014;15:329-33. [Crossref] [PubMed]
- Plourde G, Pancholy SB, Nolan J, et al. Radiation exposure in relation to the arterial access site used for diagnostic coronary angiography and percutaneous coronary intervention: a systematic review and meta-analysis. Lancet 2015;386:2192-203. [Crossref] [PubMed]
- Nishanian G, Kopchok GE, Donayre CE, et al. The impact of intravascular ultrasound (IVUS) on endovascular interventions. Semin Vasc Surg 1999;12:285-99. [PubMed]
- Weisz G, Metzger DC, Caputo RP, et al. Safety and feasibility of robotic percutaneous coronary intervention: PRECISE (Percutaneous Robotically-Enhanced Coronary Intervention) Study. J Am Coll Cardiol 2013;61:1596-600. [Crossref] [PubMed]
- Wilson SM, Prasan AM, Virdi A, et al. Real-time colour pictorial radiation monitoring during coronary angiography: effect on patient peak skin and total dose during coronary angiography. Eurointervention 2016;12:e939-47. [Crossref] [PubMed]
- Vargas A, Shroff AR, Vidovich MI. Reporting of radiation exposure in contemporary interventional cardiology trials. Catheter Cardiovasc Interv 2012;80:570-4. [Crossref] [PubMed]