
Fluid resuscitation in sepsis: the great 30 mL per kg hoax
Introduction
An aggressive approach to fluid resuscitation in patients with sepsis is recommended by international guidelines and is considered the cornerstone of treatment (1). This approach is based on historical concepts and the theory that septic shock is a form of hypovolemic shock characterized by tissue hypoperfusion (2). The surviving sepsis campaign (SSC) recommendation to “rapidly administer a minimum of 30 mL/kg crystalloid for hypotension or lactate ≥4 mmol/L” is a “strong recommendation, with a low quality of evidence” (1). Indeed, this strong recommendation is based largely on expert opinion with minimal supporting clinical data. In addition to the lack of credible data demonstrating the benefit of such a strategy, recent studies have demonstrated the potential harms with such an approach. Furthermore, results from experimental, observational and randomized clinical studies strongly suggest improved outcomes with a more restrictive approach to fluid resuscitation (2-5).
Where did the 30 mL/kg come from?
The strong recommendation that septic patients with hypotension or an elevated blood lactate concentration are required to receive at least 30 mL/kg of intravenous crystalloid within 3 hours of presentation, was a new recommendation in the fourth edition of the SSC guidelines, published in 2016 (6). Alarmingly, in the most recent revision of the SSC guidelines, the 3- and 6-h bundles have been combined into a single “1-hour bundle” with the requirement to initiate (fluid) resuscitation immediately in all patients without exception (1).
Previous versions of the SSC guidelines recommended a quantitative resuscitation protocol, based entirely on the early goal-directed therapy (EGDT) study published by Rivers et al. (7). This protocol required early and aggressive fluid resuscitation to achieve a central venous pressure (CVP) greater than 8 mmHg and central venous oxygen saturation (ScvO2) greater than 70%. EDGT and the overwhelming endorsement by the SSC ushered in an era of aggressive fluid resuscitation which persists to this day. This approach inevitably leads to massive fluid overload. EGDT was subsequently debunked in three large multicentre randomized controlled trials (8,9), however, this failed approach seems to have taken on a life of its own.
The justification provided by the SSC to support the fixed dose of 30 mL/kg in all patients is that "although little literature includes controlled data to support this volume of fluid, recent interventional studies have described this as usual practice in the early stages of resuscitation, and observational evidence is supportive” (1). The interventional studies that the guideline reference, are the average volumes of pre-randomization fluid given to patients in the PROCESS, ARISE and PROMISE trials (10-12). There is an obvious and fundamental issue with this type of circular reasoning. One describes current practice, for which there is no good evidence, and then produces a strong guideline recommendation for this current practice. The fact that clinicians on average administered 30 mL/kg of intravenous fluid before randomization was likely based on the results of the Rivers study, before the results of the 3 interventional studies provided evidence to clinicians that liberal fluid resuscitation does not improve patient-centred outcomes. It is also noteworthy that the reported mortality in the intervention group from the Rivers study was around 40%, which corresponds with the historical mortality at that time. The reported mortality of the control group however was around 60% (28-days and in-hospital), which could be considered as an excess mortality of 50% in the control group when compared to the historical mortality at that time.
The SSC guideline provides additional observational data, published by the same authors, which allegedly supports the strong recommendation for aggressive fluid resuscitation. Data from the International Multicentre Prevalence Study on Sepsis (the IMPreSS study), demonstrated that overall compliance with the SSC bundle was low, however, patients whose care was compliant with all of the recommendations had a 40% reduction in hospital mortality (13). In addition, Levy et al. demonstrated that increased compliance with the SSC bundle was associated with a mortality reduction (14). Strong conclusions based on these uncontrolled, longitudinal observational studies are fraught with pitfalls. It is important to emphasize that both studies did not analyse the effect of fluid resuscitation as an independent element of the bundle. Additionally, the association between bundle compliance and mortality is fraught with potential confounding. It is conceivable that increased bundle compliance is a marker of improved process of care or increased staffing levels while bundle non-compliance may be correlated with increased patient complexity or may be uncontrolled for co-morbidities. In addition, changing definitions of sepsis over time with increased enrolment of less sick patients likely had a dramatic effect on outcomes.
In a large study that analysed the independent effect of the fluid bolus (30 mL/kg) as a discreet element of the sepsis bundle, rapid completion of the fluid bolus had no effect on the outcome (15). In this study which analysed 26,978 patients, the time to completion of the fluid bolus was not associated with in-hospital mortality (odds ratio of 1.01 per hour; 95% CI, 0.99 to 1.02; P=0.21). Furthermore, patients in whom the fluid bolus was completed between 6 and 12 hours had a similar risk of death to patients in whom the bolus was completed in 6 hours (odds ratio of 1.02; 95% CI, 0.92 to 1.14; P=0.65). A recent large, severity adjusted observational study demonstrated that on average the total amount of fluid administered to patients with severe sepsis and septic shock during the first hospital day was considerably less than that recommended by the SSC guidelines (16). This real-world study performed in the USA emphasizes that thoughtful clinicians follow a much more prudent approach to fluid administration than recommended by the SSC guidelines. However, in this study patients who received more than 5 L of fluid during the first hospital day had a significantly increased risk of death.
Other issues with the 30 mL/kg recommendation
Aside from the fact that there is no good evidence for the strong recommendation to administer 30 mL/kg intravenous fluid to patients with septic shock, are there any other issues with this recommendation. Firstly, the SSC guidelines do not state whether clinicians should use actual body weight, predicted body weight or ideal body weight. Which weight metric clinician’s use will have a significant impact on the amount of fluid prescribed, especially in the extremes of weight (e.g., severe underweight, severe overweight). For example, when using actual body weight in a patient weighing 150 kg, a fluid bolus of 4,500 mL should be administered. If one does not adhere to this guideline and one happens to practice in the State of New York (USA), there is a real risk of being sued for malpractice (non-adherence to the so-called SEP-1 mandate). If one does adhere to the guideline, one still risks litigation for causing hypervolemia-associated morbidity or mortality, especially in a patient with concomitant cardiac or kidney failure. Regardless, a relevant practical issue is that height and weight data are unlikely to be available to the clinician who is treating the patient with septic shock (17). When weight data are not immediately available and weight-based fluid resuscitation is recommended, it is likely that clinicians will estimate the patient’s weight. Unfortunately, estimations of patients’ weight made by intensive care unit staff, regardless of whether this is medical or nursing staff, have been shown to be notoriously unreliable (18,19).
Second, the recommendation of a fixed resuscitation volume of 30 mL/kg in all patients with septic shock is an example of a “one-size-fits-all” approach. This approach contradicts the current paradigm that medical treatments, including fluid administration, should be individualized and personalized (9,20). In one study conducted in 2 hospitals in the USA, the validity of this “one-size-fits-all” approach to the management of patients with septic shock was questioned (21). In this study, 47.3% of 1027 septic shock patients met the 6-hour 30 mL/kg fluid requirement. Compliance was lower in patients with chronic kidney disease (42.3%), heart failure (40.9%) and those with chronic liver disease (38.5%). When adjusting for relevant covariates, compliance with the fluid requirement was not associated with in-hospital mortality (OR 1.03, 95% CI: 0.76–1.41). Finally, there is emerging evidence that blindly following the SSC protocols is potentially harmful to patients (22). Potential patient harm caused by fluid resuscitation will be discussed in more detail below.
Is there actually any evidence for fluid resuscitation in sepsis?
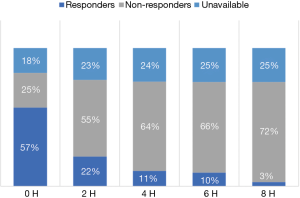
The history of fluid resuscitation as well as the preclinical and clinical evidence, or rather lack thereof, for fluid resuscitation as treatment for severe sepsis and septic shock has been reviewed in detail elsewhere (2). In short, the assumed effectiveness of the fluid treatment was based on an incorrect and incomplete understanding of the pathophysiology of sepsis. In this hypoperfusion-centric paradigm, the case for organ hypoperfusion was based on the presence of an increased blood lactate concentration, oliguria, hepatic dysfunction and altered mental state, amongst others. It was assumed that these findings were a consequence of organ hypoperfusion and that fluid resuscitation would result in clinically relevant increases in cardiac output which would then reverse the pathological organ hypoperfusion. At multiple levels this reasoning is overly simplistic and mostly wrong. There is emerging evidence that cerebral, cardiac, renal and hepatic dysfunction in sepsis is largely caused by bioenergetic failure rather than microcirculatory dysfunction and impaired organ perfusion. This is best demonstrated in the kidney where renal blood flow is usually maintained despite oliguria and impaired renal function. Furthermore, it is essential to recognize that the septic heart responds poorly to fluid loading and that aggressive fluid administration may paradoxically further impair cardiac function. In patients with sepsis the Frank-Starling (or cardiac function curve) is shifted downwards and to the right, with the septic heart showing a limited response to fluid loading. Ognibene and colleagues demonstrated this finding over 25 years ago (23). In this study, patients with septic shock demonstrated a minimal increase in end-diastolic volume and stroke volume following a fluid challenge. Furthermore, due to alterations in ventricular compliance large volume fluid resuscitation will cause large increases in filling pressures leading to pulmonary edema (high left atrial pressure) and increased hepatic and renal venous pressures (high right atrial pressure) with consequent organ dysfunction. Additionally, emerging data suggests that at presentation only about 50% of patients with septic shock (who are fluid naive) will demonstrate a clinically significant increase in stroke volume in response to a fluid bolus (i.e., are fluid responsive) (24). Furthermore, this study demonstrated that those patients who were initially fluid responders rapidly become non-responsive to fluid challenges (Figure 1). It is therefore important to emphasize that in most patient’s aggressive fluid loading will have minimal hemodynamic benefits but will come at the cost of significant “downstream” harmful effects. There is, however, a group of septic patients who are truly hypovolemic (dehydrated). These are usually elderly patients who have been sick for some time with decreased oral intake and/or nausea and vomiting. In these patients, limited fluid administration will usually correct the patients’ hypotension and tachycardia. However, fluids alone will not reverse the hemodynamic instability in patients with more severe sepsis; in these patients aggressive fluid administration will likely worsen the vasodilatory shock and myocardial dysfunction and increase the microcirculatory injury with increased organ edema and organ dysfunction (25). In summary, the evidence supporting fluid resuscitation as an effective and safe treatment for sepsis is essentially non-existent (2).
Emerging evidence of harm associated with fluid resuscitation in sepsis
There are two fundamental mechanisms by which aggressive fluid administration may be harmful to the septic patient. The first relates to the direct effects of large volume resuscitation on cardiovascular function, paradoxically worsening shock. The second mechanism is related to the deleterious effects of volume overload on organ function. While balanced crystalloids are generally regarded as the fluid of choice, the specific benefits and harms of different types of resuscitation fluids will not be reviewed in this paper.
Cardiovascular dysfunction associated with fluid bolus therapy
As mentioned earlier, fluid resuscitation using fluid bolus therapy is regarded as the initial intervention of choice in patients with sepsis induced hypotension and in patients with an elevated lactate concentration. The stated goals of fluid resuscitation include a CVP >8 mmHg, a mean arterial pressure >65 mmHg with an improvement in urine output (6). Improvement of these hemodynamic parameters is presumed to indicate improved tissue perfusion which would then result in better clinical outcomes. However, despite an apparent initial improvement, cardiovascular dysfunction and outcomes appear to worsen.
In the landmark “fluid expansion as supportive therapy (FEAST)” trial, 3,141 children with severe sepsis were randomized to receive fluid resuscitation with either 40 mL/kg of 0.9% saline, 4% albumin or no-volume resuscitation (3). The trial was stopped early due to a 40% increased mortality in the fluid arms. Subgroup analysis failed to identify any group of patients that benefitted from the large fluid volume resuscitation strategy. Surprisingly, the cause of the increased mortality in the patients’ that received fluid was not related to complications associated with fluid overload but rather due to delayed cardiovascular collapse producing refractory shock (26). Following the FEAST trial, a randomized controlled trial conducted in Zambia, randomized 209 adult patients with septic shock to a 6-hour sepsis protocol compared to usual care (4). The 6-hour sepsis protocol included a 2-liter fluid bolus administered within 1 hour of enrollment, followed by an additional 2-liter over the subsequent 4 hours (very similar to the SSC guideline). Despite receiving a significantly greater volume of fluid, patients in the protocol group required greater use of vasopressor agents. The 28-day survival was significantly better in the usual group as compared to the protocol group (58% vs. 36%, P=0.02). These two randomized controlled trials highlight the significant harm associated with an aggressive fluid resuscitation strategy.
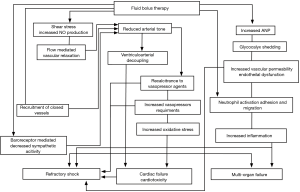
In order to better understand the disturbing findings of these pivotal clinical studies, Byrne and colleagues performed an ovine experimental study which compared an early fluid resuscitation strategy versus an early vasopressor, no-fluid resuscitation approach (27). This study was performed using a validated hyperdynamic sheep model of sepsis (28). After the induction of endotoxemic shock, the animals received fluid resuscitation with 40 mL/kg of 0.9% saline given over 1 hour (like the FEAST study) followed by vasopressor support or hemodynamic support with protocolized noradrenaline (norepinephrine) and vasopressin but without a fluid bolus. As expected, the fluid resuscitated animals had an increase in cardiac output immediately following administration of the fluid bolus. While the mean arterial blood pressure increased following fluid administration, the extent of increase was small compared to the increase in cardiac output due to a simultaneous fall in the systemic vascular resistance. In keeping with the observations from the pivotal randomized controlled trials, the animals in the fluid resuscitation group required considerably more noradrenaline to maintain the same mean arterial pressure in the 12 hours after resuscitation. These findings suggested that large volume fluid resuscitation induced vasodilation and resistance to vasopressor agents. Importantly, there was evidence of increased myocardial injury (troponin levels) and damage to the endothelial glycocalyx (hyaluronan levels) in the fluid resuscitation animals. In summary, fluid resuscitation has the potential unintended consequence that it may worsen shock. The potential pathways that large volume fluid resuscitation may cause cardiovascular dysfunction are discussed below and summarized in Figure 2.
Fluid resuscitation induced vasodilation
Several experimental and clinical studies have demonstrated that large volume fluid resuscitation causes vasodilation. Furthermore, experimental studies suggest that fluid administration may transform the initial non-resuscitated hypodynamic profile into a hyperdynamic state (29-31). Monge García et al. studied the effects of a fluid bolus on arterial load in 81 septic patients (32). In this study only 44% of volume responsive patients had an increase in mean arterial pressure. Fluid resuscitation resulted in a decrease in systemic vascular resistance that was most marked amongst the patients whose cardiac output increased. Similarly, Pierrakos et al. found that in preload responsive patients fluid resuscitation resulted in a significant decrease in systemic vascular resistance (33). In another experimental study, fluid bolus administration decreased dynamic arterial elastance, with no relationship between the changes in cardiac output and mean arterial pressure (34). Several explanations have been hypothesized to explain fluid bolus induced vasodilation. The rapid infusion of a large volume of fluid may attenuate the baroreflex-mediated vasoconstriction in response to hypovolemia (32). Fluid administration may recruit previously closed vessels, thereby reducing arterial resistance. A fluid bolus will increase blood flow velocity and endothelial shear stress (31). Increased endothelial shear stress may result in flow mediated vascular relaxation secondary to the release of endothelial nitric oxide (35). In addition, shear stress results in integrin-mediated release of fibroblast growth factor 2, which is believed to play a role in endothelium-dependent control of vascular tone (36). Finally, the rapid administration of large volumes of fluid increase cardiac filling pressures and the release of natriuretic peptides. Natriuretic peptides are potent vasodilators, acting via cGMP pathways (like nitric oxide).
Fluid resuscitation associated cardiotoxicity
Large volume resuscitation may lead to cardiovascular collapse by direct cardiotoxicity. In the ovine study by Byrne and co-workers, animals in the fluid resuscitation group developed impaired myocardial contractility with evidence of myocardial injury (27). The causation of fluid induced cardiotoxicity is uncertain; however, several potential explanations have been suggested. These include increased myocardial edema, mitochondrial oxidative stress, microvascular thrombi and increased sarcolemma membrane permeability (37,38). Furthermore, the requirement for increased doses of vasopressors may play a pathogenetic role. Catecholamines have been demonstrated to increase myocardial oxidative stress (39). Increased oxidative stress may play a central role in the pathogenesis of sepsis induced myocardial dysfunction (40).
Effects of fluid resuscitation on the glycocalyx
Degradation of the endothelial glycocalyx is an early finding in sepsis (41). Large volume fluid administration may potentiate damage to the glycocalyx, especially when the fluid bolus is given rapidly. Circulating levels of hyaluronan are frequently used as a marker of degradation of the glycocalyx barrier (42-45). Increased levels of hyaluronan have been reported following intravenous fluid administration, suggesting fluid induced damage to the glycocalyx (46). Shedding of the glycocalyx may be mediated by the release of atrial natriuretic peptide in response to hypervolemia (45). In experimental models, the exogenous administration of physiological levels of atrial natriuretic peptide has been demonstrated to cause shedding of the glycocalyx with increased vascular permeability (47). In the study by Byrne and colleagues, animals assigned to the fluid resuscitation group demonstrated a prolongation of endotoxemia-induced release of circulating atrial natriuretic peptide, followed by an increased rate of hyaluronic acid shedding into the blood (27). A recent study demonstrated that the volume of fluid administered during the resuscitation of septic patients was independently associated with the degree of glycocalyx degradation (48). In this study, the degree of injury to the glycocalyx was associated with in-hospital mortality.
Inflammatory effects of fluid resuscitation
The approach to fluid resuscitation may impact the circulating profile of inflammatory mediators (49). In an experimental human endotoxemia model, prehydration shifted the cytokine pattern toward a more anti-inflammatory state which was associated with reduced clinical features of sepsis (50). It has been proposed that resuscitation fluids may have dose-dependent pro-inflammatory properties (51,52). Furthermore, the composition of the resuscitation fluid may impact the inflammatory response. In patients with septic shock administration of hypertonic as compared to isotonic fluids alters the expression of genes that are implicated in leukocyte-endothelial interactions which influence microvascular function and capillary permeability (53).
Harm caused by fluid overload
The degree of volume overload is best assessed by calculating the percent of fluid accumulation. The percentage of fluid accumulation is calculated by dividing the cumulative fluid balance in litres by the patient’s baseline body weight, multiplied by 100% (54). Volume overload is defined as a fluid accumulation of 10% or greater (54). Increasing fluid overload induces a vicious cycle of interstitial oedema and organ dysfunction. There is now indisputable evidence that volume overload results in multi-organ dysfunction with adverse patient outcomes (5,55-59). Due to the curvilinear ventricular pressure-volume relationship, atrial pressures increase rapidly as the patient reaches the plateau of his/her Frank-Starling curve. Increased atrial pressure increases pulmonary and venous hydrostatic pressures which combined with the increased release of natriuretic peptides, causes a shift of fluid into the interstitial space with increasing pulmonary and tissue edema. Tissue oedema distorts tissue architecture, impedes capillary blood flow and lymphatic drainage, disturbs cell-cell interactions and impairs oxygen and metabolite diffusion (60,61). Furthermore, increased right atrial pressure (CVP) is transmitted retrograde increasing venous pressure in vital organs. The increased venous pressure has profound effects on microcirculatory flow and organ function (62). The kidney is particularly vulnerable to increased venous pressure, which leads to increased renal subscapular pressure and reduced renal blood flow (60). In addition, increased renal interstitial pressure may collapse intrarenal collecting lymphatics compromising lymphatic flow (63). These effects result in oliguria with a marked decrease in renal function.
Fluid overload can be caused by overly aggressive initial and on-going fluid resuscitation but also by maintenance fluid therapy and “fluid creep” (64). Furthermore, the type of fluid used (hypotonic vs. isotonic) may have an impact on salt and water retention (65). Fluid overload affects the function of all the major organ systems. Aggressive fluid resuscitation has been well established to be a major risk factor for secondary intra-abdominal hypertension which in turn is associated with acute kidney injury, hepatic and respiratory dysfunction, multi-organ failure and death (66-69). Below is a list of the potential detrimental effects of fluid overload on end-organ function:
- Central nervous system: impaired cognition, delirium, increased intracranial, intra-orbital and intra-ocular pressure, cerebral oedema, intracranial hypertension and diminished cerebral perfusion pressure.
- Respiratory system: pulmonary oedema, pleural effusions, increased chest wall elastance, increased extravascular lung water, hypercarbia, hypoxia, decreased lung volumes (due to increased intraabdominal pressure), increased work of breathing, prolonged weaning with a prolonged duration of mechanical ventilation.
- Cardiovascular system: myocardial oedema, impaired contractility with myocardial depression leading to a reduced ejection fraction and decreased cardiac output with concomitant diastolic dysfunction and increased filling pressures.
- Renal system: increased renal venous and interstitial pressure, decreased renal blood flow and glomerular filtration rate, increased renal vascular resistance, renal venous congestion, uraemia, salt and water retention and renal compartment syndrome.
- Gastrointestinal system: bowel oedema, diminished hepato-splanchnic perfusion, decreased bowel motility with ileus and malabsorption, increased intestinal permeability and bacterial translocation, ascites formation, increased intra-abdominal pressure with and decreased abdominal perfusion pressure, abdominal hypertension and abdominal compartment syndrome.
- Hepatic system: diminished liver perfusion, hepatic venous congestion, transaminitis, decreased hepatic synthetic function and a hepatic compartment syndrome.
In patients with sepsis a conservative fluid strategy will likely improve patient outcomes, by avoiding the complications listed above (70). To date multiple studies have been published all demonstrating that aggressive fluid resuscitation leading to fluid overload is associated with increased complications and death. Furthermore, there are no published studies demonstrating that the “under-resuscitation” of septic patients leads to worse outcomes, indeed the opposite is likely true. This is best demonstrated by the FEAST study, where “no-fluid resuscitation” was associated with reduced mortality (3). The overwhelming preponderance of high-quality evidence demonstrates that septic patients are poorly response to fluid resuscitation and that overly aggressive fluid administration increases the risk of death. Furthermore, there is no evidence (apart from “expert opinion”) to blindly administer a 30m/kg fluid bolus to septic patients with hypotension or an increased blood lactate concentration. Such an approach is likely harmful in most patients and one of the greatest hoaxes of modern medicine.
Several randomized controlled trials are currently underway comparing a liberal with a more conservative approach to fluid resuscitation in patients with septic shock (NCT03434028, NCT03668236). These trials are rather unfortunate in that the use a binary approach to fluid resuscitation. These studies do not stratify patients based on their fluid responsiveness, left and right ventricular function, the degree of hemodynamic derangement nor by the patient’s comorbidities. These factors are essential to consider in the resuscitation of critically ill patients. Consequently, these studies are unlikely to inform the thoughtful clinician on how to best manage fluid administration in critically ill septic patients.
We believe that the approach to fluid and vasopressor resuscitation in the critically ill septic patient should be individualized and based on the patients unique hemodynamics and clinical features. Blindly following a simplistic inflexible protocol will inevitably harm patients. Emerging data suggests that a hemodynamically guided and conservative approach to fluid therapy in patients with sepsis will reduce morbidity and improve patient outcomes. Furthermore, norepinephrine should be initiated early in patients with septic shock. Septic patients should receive just the right amount of fluid and not a drop more. The requirement for de-resuscitation implies that the patient was over-resuscitated with fluid in the first instance. We follow the approach of Dr. Thomas Latta, the father of fluid resuscitation, who in 1832 “inserted a tube into the basilic vein and injected ounce after ounce of fluid, closely observing the patient” (71). We endorse a similar approach and recommend administering a 500 ml bolus of a balanced crystalloid solution, closely monitoring the patients’ response to the bolus before contemplating further boluses of fluid.
Conclusions
Aggressive fluid resuscitation has long been considered the cornerstone of treatment for septic shock. There is however no scientific evidence to support this treatment approach, and indeed the preponderance of evidence suggests that such an approach is harmful. Potential mechanisms of harm include cardiovascular collapse associated with vasodilation, cardiotoxicity and damage to the endothelial glycocalyx. Furthermore, fluid overload results in widespread tissue edema leading to organ dysfunction. We suggest that an individualized, hemodynamically guided and fluid restricted approach to fluid therapy will improve the outcomes of patients with severe sepsis and septic shock.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Levy MM, Evans LE, Rhodes A. The Surviving Sepsis Campaign Bundle: 2018 update. Intensive Care Med 2018;44:925-8. [Crossref] [PubMed]
- Byrne L, Van Haren F. Fluid resuscitation in human sepsis: Time to rewrite history? Ann Intensive Care 2017;7:4. [Crossref] [PubMed]
- Maitland K, Kiguli S, Opoka RO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med 2011;364:2483-95. [Crossref] [PubMed]
- Andrews B, Semler MW, Muchemwa L, et al. Effect of an Early Resuscitation Protocol on In-hospital Mortality Among Adults With Sepsis and Hypotension: A Randomized Clinical Trial. JAMA 2017;318:1233-40. [Crossref] [PubMed]
- Boyd JH, Forbes J, Nakada TA, et al. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med 2011;39:259-65. [Crossref] [PubMed]
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017;43:304-77. [Crossref] [PubMed]
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001;345:1368-77. [Crossref] [PubMed]
- Angus DC, Barnato AE, Bell D, et al. A systematic review and meta-analysis of early goal-directed therapy for septic shock: the ARISE, ProCESS and ProMISe Investigators. Intensive Care Med 2015;41:1549-60. [Crossref] [PubMed]
- Vandervelden S, Malbrain ML. Initial resuscitation from severe sepsis: one size does not fit all. Anaesthesiol Intensive Ther 2015;47:s44-55. [Crossref] [PubMed]
- Peake SL, Delaney A, Bailey M, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014;371:1496-506. [Crossref] [PubMed]
- Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 2015;372:1301-11. [Crossref] [PubMed]
- ProCESS Investigators, Yealy DM, Kellum JA, et al. A Randomized Trial of Protocol-Based Care for Early Septic Shock. N Engl J Med 2014;370:1683-93. [Crossref] [PubMed]
- Rhodes A, Phillips G, Beale R, et al. The Surviving Sepsis Campaign bundles and outcome: results from the International Multicentre Prevalence Study on Sepsis (the IMPreSS study). Intensive Care Med 2015;41:1620-8. [Crossref] [PubMed]
- Levy MM, Rhodes A, Phillips GS, et al. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Crit Care Med 2015;43:3-12. [Crossref] [PubMed]
- Seymour CW, Gesten F, Prescott HC, et al. Time to Treatment and Mortality during Mandated Emergency Care for Sepsis. N Engl J Med 2017;376:2235-44. [Crossref] [PubMed]
- Marik PE, Linde-Zwirble WT, Bittner EA, et al. Fluid administration in severe sepsis and septic shock, patterns and outcomes: an analysis of a large national database. Intensive Care Med 2017;43:625-32. [Crossref] [PubMed]
- Hendershot KM, Robinson L, Roland J, et al. Estimated height, weight and body mass index: Implications for research and patient safety. J Am Coll Surg 2006;203:887-93. [Crossref] [PubMed]
- Maskin LP, Attie S, Setten M, et al. Accuracy of weight and height estimation in an intensive care unit. Anaesth Intensive Care 2010;38:930-4. [Crossref] [PubMed]
- Bloomfield R, Steel E, MacLennan G, et al. Accuracy of weight and height estimation in an intensive care unit: Implications for clinical practice and research. Crit Care Med 2006;34:2153-7. [Crossref] [PubMed]
- van Haren F. Personalised fluid resuscitation in the ICU: still a fluid concept? Crit Care 2017;21:313. [Crossref] [PubMed]
- Truong TN, Dunn AS, McCardle K, et al. Adherence to fluid resuscitation guidelines and outcomes in patients with septic shock: Reassessing the "one-size-fits-all" approach. J Crit Care 2019;51:94-8. [Crossref] [PubMed]
- Marik PE, Malbrain M. The SEP-1 quality mandate may be harmful: How to drown a patient with 30 mL per kg fluid! Anaesthesiol Intensive Ther 2017;49:323-8. [Crossref] [PubMed]
- Ognibene FP, Parker MM, Natanson C, et al. Depressed left ventricular performance. Response to volume infusion in patients with sepsis and septic shock. Chest 1988;93:903-10. [Crossref] [PubMed]
- Hernández G, Ospina-Tascon GA, Damiani LP, et al. Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs Serum Lactate Levels on 28-Day Mortality Among Patients With Septic Shock: The ANDROMEDA-SHOCK Randomized Clinical Trial. JAMA 2019;321:654-64. [Crossref] [PubMed]
- Rehberg S, Yamamoto Y, Sousse L, et al. Selective V(1a) agonism attenuates vascular dysfunction and fluid accumulation in ovine severe sepsis. Am J Physiol Heart Circ Physiol 2012;303:H1245-54. [Crossref] [PubMed]
- Maitland K, George EC, Evans JA, et al. Exploring mechanisms of excess mortality with early fluid resuscitation: insights from the FEAST trial. BMC Med 2013;11:68. [Crossref] [PubMed]
- Byrne L, Obonyo NG, Diab SD, et al. Unintended Consequences; Fluid Resuscitation Worsens Shock in an Ovine Model of Endotoxemia. Am J Respir Crit Care Med 2018;198:1043-54. [Crossref] [PubMed]
- Byrne L, Obonyo NG, Diab S, et al. An Ovine Model of Hyperdynamic Endotoxemia and Vital Organ Metabolism. Shock 2018;49:99-107. [Crossref] [PubMed]
- Cholley BP, Lang RM, Berger DS, et al. Alterations in systemic arterial mechanical properties during septic shock: role of fluid resuscitation. Am J Physiol 1995;269:H375-84. [PubMed]
- Ricard-Hibon A, Losser MR, Kong R, et al. Systemic pressure-flow reactivity to norepinephrine in rabbits: impact of endotoxin and fluid loading. Intensive Care Med 1998;24:959-66. [Crossref] [PubMed]
- Losser MR, Forget AP, Payen D. Nitric oxide involvement in the hemodynamic response to fluid resuscitation in endotoxic shock in rats. Crit Care Med 2006;34:2426-31. [Crossref] [PubMed]
- Monge García MI, González PG, Romero MG, et al. Effects of fluid administration on arterial load in septic shock patients. Intensive Care Med 2015;41:1247-55. [Crossref] [PubMed]
- Pierrakos C, Velissaris D, Scolletta S, et al. Can changes in arterial pressure be used to detect changes in cardiac index during fluid challenge in patients with septic shock? Intensive Care Med 2012;38:422-8. [Crossref] [PubMed]
- Monge García MI, Guijo Gonzalez P, Gracia Romero M, et al. Effects of arterial load variations on dynamic arterial elastance: an experimental study. Br J Anaesth 2017;118:938-46. [Crossref] [PubMed]
- Pohl U, De Wit C, Gloe T. Large arterioles in the control of blood flow: role of endothelium-dependent dilation. Acta Physiol Scand 2000;168:505-10. [Crossref] [PubMed]
- Hennig T, Mogensen C, Kirsch J, et al. Shear stress induces the release of an endothelial elastase: role in integrin alpha(v)beta(3)-mediated FGF-2 release. J Vasc Res 2011;48:453-64. [Crossref] [PubMed]
- Maeder M, Fehr T, Rickli H, et al. Sepsis-associated myocardial dysfunction: diagnostic and prognostic impact of cardiac troponins and natriuretic peptides. Chest 2006;129:1349-66. [Crossref] [PubMed]
- Bessière F, Khenifer S, Dubourg J, et al. Prognostic value of troponins in sepsis: a meta-analysis. Intensive Care Med 2013;39:1181-9. [Crossref] [PubMed]
- Neri M, Cerretani D, Fiaschi AI, et al. Correlation between cardiac oxidative stress and myocardial pathology due to acute and chronic norepinephrine administration in rats. J Cell Mol Med 2007;11:156-70. [Crossref] [PubMed]
- Haileselassie B, Su E, Pozios I, et al. Myocardial oxidative stress correlates with left ventricular dysfunction on strain echocardiography in a rodent model of sepsis. Intensive Care Med Exp 2017;5:21. [Crossref] [PubMed]
- Henrich M, Gruss M, Weigand MA. Sepsis-induced degradation of endothelial glycocalix. ScientificWorldJournal 2010;10:917-23. [Crossref] [PubMed]
- von Geldern TW, Budzik GP, Dillon TP, et al. Atrial natriuretic peptide antagonists: biological evaluation and structural correlations. Mol Pharmacol 1990;38:771-8. [PubMed]
- Bruegger D, Jacob M, Rehm M, et al. Atrial natriuretic peptide induces shedding of endothelial glycocalyx in coronary vascular bed of guinea pig hearts. Am J Physiol- Heart Circ Physiol 2005;289:H1993-9. [Crossref] [PubMed]
- Chen C, Chappell D, Annecke T, et al. Sevoflurane mitigates shedding of hyaluronan from the coronary endothelium, also during ischemia/reperfusion: an ex vivo animal study. Hypoxia 2016;4:81-90. [PubMed]
- Chappell D, Bruegger D, Potzel J, et al. Hypervolemia increases release of atrial natriuretic peptide and shedding of the endothelial glycocalyx. Crit Care 2014;18:538. [Crossref] [PubMed]
- Berg S, Engman A, Hesselvik JF, et al. Crystalloid infusion increases plasma hyaluronan. Crit Care Med 1994;22:1563-7. [Crossref] [PubMed]
- Jacob M, Saller T, Chappell D, et al. Physiological levels of A-, B-and C-type natriuretic peptide shed the endothelial glycocalyx and enhance vascular permeability. Basic Res Cardiol 2013;108:347. [Crossref] [PubMed]
- Hippensteel JA, Uchimido R, Tyler PD, et al. Intravenous fluid resuscitation is associated with septic endothelial glycocalyx degradation. Crit Care 2019;23:259. [Crossref] [PubMed]
- Rivers EP, Kruse JA, Jacobsen G, et al. The influence of early hemodynamic optimization on biomarker patterns of severe sepsis and septic shock. Crit Care Med 2007;35:2016-24. [Crossref] [PubMed]
- Dorresteijn MJ, van Eijk LT, Netea MG, et al. Iso-osmolar prehydration shifts the cytokine response towards a more anti-inflammatory balance in human endotoxemia. J Endotoxin Res 2005;11:287-93. [Crossref] [PubMed]
- Rhee P, Wang D, Ruff P, et al. Human neutrophil activation and increased adhesion by various resuscitation fluids. Crit Care Med 2000;28:74-8. [Crossref] [PubMed]
- Lee SH, Seo E-H, Park HJ, et al. The effects of crystalloid versus synthetic colloid in vitro on immune cells, co-cultured with mouse splenocytes. Scientific Reports 2018;8:4794. [Crossref] [PubMed]
- van Haren FM, Sleigh J, Cursons R, et al. The effects of hypertonic fluid administration on the gene expression of inflammatory mediators in circulating leucocytes in patients with septic shock: a preliminary study. Ann Intensive Care 2011;1:44. [Crossref] [PubMed]
- Vincent JL, Pinsky MR. We should avoid the term "fluid overload". Crit Care 2018;22:214. [Crossref] [PubMed]
- Malbrain ML, Marik PE, Witters I, et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther 2014;46:361-80. [Crossref] [PubMed]
- Sadaka F, Juarez M, Naydenov S, et al. Fluid resuscitation in septic shock: the effect of increasing fluid balance on mortality. J Intensive Care Med 2014;29:213-7. [Crossref] [PubMed]
- Smith SH, Perner A. Higher vs. lower fluid volume for septic shock: clinical characteristics and outcome in unselected patients in a prospective, multicenter cohort. Crit Care 2012;16:R76. [Crossref] [PubMed]
- Samoni S, Vigo V, Resendiz LI, et al. Impact of hyperhydration on the mortality risk in critically ill patients admitted in intensive care units: comparison between bioelectrical impedance vector analysis and cumulative fluid balance recording. Crit Care 2016;20:95. [Crossref] [PubMed]
- Prowle JR, Echeverri JE, Ligabo EV, et al. Fluid balance and acute kidney injury. Nat Rev Nephrol. 2010;6:107-15. [Crossref] [PubMed]
- Prowle JR, Kirwan CJ, Bellomo R. Fluid management for the prevention and attenuation of acute kidney injury. Nat Rev Nephrol. 2014;10:37-47. [Crossref] [PubMed]
- Hilton AK, Bellomo R. A critique of fluid bolus resuscitation in severe sepsis. Crit Care 2012;16:302. [Crossref] [PubMed]
- Vellinga NA, Ince C, Boerma EC. Elevated central venous pressure is associated with impairment of microcirculatory blood flow in sepsis: a hypothesis generating post hoc analysis. BMC Anesthesiol 2013;13:17. [Crossref] [PubMed]
- Rohn DA, Stewart RH, Elk JR, et al. Renal lymphatic function following venous pressure elevation. Lymphology 1996;29:67-75. [PubMed]
- Van Regenmortel N, Verbrugghe W, Roelant E, et al. Maintenance fluid therapy and fluid creep impose more significant fluid, sodium, and chloride burdens than resuscitation fluids in critically ill patients: a retrospective study in a tertiary mixed ICU population. Intensive Care Med 2018;44:409-17. [Crossref] [PubMed]
- Van Regenmortel N, Hendrickx S, Roelant E, et al. 154 compared to 54 mmol per liter of sodium in intravenous maintenance fluid therapy for adult patients undergoing major thoracic surgery (TOPMAST): a single-center randomized controlled double-blind trial. Intensive Care Med 2019;45:1422-32. [Crossref] [PubMed]
- Malbrain ML, Chiumello D, Pelosi P, et al. Incidence and prognosis of intraabdominal hypertension in a mixed population of critically ill patients: a multiple-center epidemiological study. Crit Care Med 2005;33:315-22. [Crossref] [PubMed]
- Kirkpatrick AW, Roberts DJ, De Waele J, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med 2013;39:1190-206. [Crossref] [PubMed]
- Dalfino L, Tullo L, Donadio I, et al. Intra-abdominal hypertension and acute renal failure in critically ill patients. Intensive Care Med 2008;34:707-13. [Crossref] [PubMed]
- Diebel LN, Wilson RF, Dulchavsky SA, et al. Effect of increased intra-abdominal pressure on hepatic arterial, portal venous, and hepatic microcirculatory blood flow. J Trauma 1992;33:279-82. [Crossref] [PubMed]
- Silversides JA, Perner A, Malbrain M. Liberal versus restrictive fluid therapy in critically ill patients. Intensive Care Med 2019;45:1440-2. [Crossref] [PubMed]
- MacGillivray N. Dr Latta of Leith: pioneer in the treatment of cholera by intravenous saline infusion. J R Coll Physicians Edinb 2006;36:80-5. [PubMed]


