
Relationship between compliance and pulmonary vascular resistance in pulmonary arterial hypertension
Introduction
Prognosis in pulmonary arterial hypertension (PAH) has classically been indexed to changes in pulmonary vascular resistance (PVR). However, as a measure of right ventricular (RV) afterload, the PVR falls short of providing a comprehensive assessment of the pulmonary arterial blood flow as it characterizes the dynamic and pulsatile nature of the pulmonary circulation by accounting mainly for opposition to continuous flow (1). Pulmonary arterial compliance (PAC), an intrinsic property of the pulmonary vasculature, has been integrated into clinical decision-making as a surrogate marker. Compliance is a measure of the change in cross sectional area or volume over change in pressure at a fixed vessel length (2).
Compliance has been shown to be an important prognostic marker in PAH in addition to PVR, and was even proven superior to others such as WHO class, right atrial pressure (RAP) and mean pulmonary artery pressure (PAP) (3). Its importance has thus far been demonstrated mostly in the early phases of pulmonary hypertension, and as such has been designated as an early marker of disease (4). The direct comparison of compliance and PVR has previously yielded a logarithmic relationship, whereby PAC × PVR is a constant called the arterial time (RC) constant which remains stable up to 1 year after treatment initiation (5,6). The primary aim of this study is to analyze the long-term stability of the PAC/PVR relationship and secondarily, to assess each PAH etiology separately from a hemodynamic and clinical standpoint.
Methods
Patient selection
The study was approved by the Mount Sinai Medical Center Institutional Review Board, and included patients diagnosed with PAH and followed at the Mount Sinai Medical Center Pulmonary Hypertension Clinic between 2008 and 2019. The diagnosis of type 1 PAH was made by right heart catheterization demonstrating a mean PAP ≥25 mmHg at rest and PVR ≥3 Wood units (WU). All patients evaluated for PAH who fulfilled WHO group I PAH criteria were included in the study, provided they had undergone two right heart catheterizations at least 1 year apart. Patients with no right heart catheterization or incomplete hemodynamic data were excluded from the study. Right heart catheterization was repeated periodically throughout the patients’ treatment course following clinical worsening or as a measure of response to medical therapy. Clinical and hemodynamic data were collected for each patient at enrollment and at each catheterization.
Clinical and hemodynamic variables
The clinical variables collected on each patient included: sex, age, PAH etiology, New York Heart Association (NYHA) functional class, and treatment regimen. Hemodynamic recording in the cardiac catherization laboratory was performed using the Siemens Sensis hemodynamic system. The institution’s electronic medical record system was queried and hemodynamic tracings of individual catheterizations were retrieved and analyzed for each patient included in the study. The hemodynamic variables assessed were heart rate (HR), cardiac output (CO) by the Fick or thermodilution method, RAP, systolic and diastolic PAP, and pulmonary capillary wedge pressure (PCWP).
Computed variables
The data collected was used to calculate PVR and PAC according to the following formulas:
PVR = (Mean PAP – PCWP)/CO
PAC = Stroke volume/PA pulse pressure = CO/[HR × (systolic PAP – diastolic PAP)]
Finally, the RC product was calculated for each set of PVR and PAC values in accordance with prior studies, with the formula (5,6):
RC = PVR × PAC
Statistical analysis
The data was analyzed using Statistical Package for the Social Sciences software (SPSS version 24). Continuous variables were compared using an independent t-test and trend analysis was performed via ANOVA. A P value of ≤0.05 was considered statistically significant.
Results
Patient characteristics
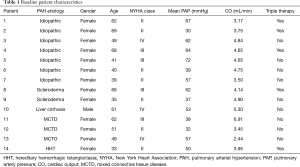
A total of 82 patients underwent right heart catheterization during the study period, of which 14 met the inclusion criteria and were included in the study. Patients were excluded due to incomplete hemodynamic data (n=46), consecutive catheterizations less than a year apart (n=18), and combined pre- and post-capillary pulmonary hypertension (n=4). The etiology of PAH in the study group was idiopathic (n=7), mixed connective tissue disease (MCTD) (n=3), scleroderma (n=2), liver cirrhosis (n=1), and hereditary hemorrhagic telangiectasia (HHT) (n=1). The mean age at first catheterization was 52±13 years, and 93% were female. At baseline, 50% of patients were classified as NYHA functional class II symptoms, 29% had NYHA class III symptoms, and 21% had NYHA class IV symptoms. CO on average was 4.31±1.09 L/min. Triple therapy was utilized in 36% of patients at the time of initial right heart catheterization; while double therapy was utilized in 36% and single therapy in 28% of patients (Table 1).
Full table
Hemodynamic assessment
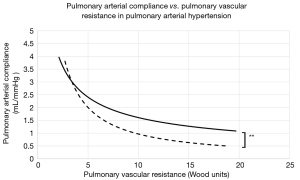
The average time between initial and final catheterizations was 3.9±2.1 years. At baseline and across all PAH groups, PVR ranged from 4.1 to 19.7 WU (mean, 9.2±8.7), while compliance ranged from 0.7 to 2.7 mL/mmHg (mean, 1.3±0.6). The mean baseline RC product was 10.65±3.5. At the end of the study period, the mean RC product was calculated at 10.05±1.9. The difference between the initial and final mean RC products was not statistically significant (P=0.61), confirming the stability of the RC product across time. Patients with PAH secondary to MCTD showed significantly higher RC constants at any given time (12.54 vs. 10.01, P<0.01). This observation was driven by a significantly higher PAC (2.59 vs. 1.62 mL/mmHg, P=0.02) at corresponding PVR values (Figure 1) when compared to other PAH etiologies (Table 2). Finally, the average mean PAP at baseline and follow-up measured 51±14 vs. 46±13 mmHg, respectively.

Full table
Clinical outcomes
The idiopathic PAH group demonstrated significant clinical and hemodynamic heterogeneity, with compliance values widely scattered around the mean, likely alluding to a wide range of underlying pathologies or stages of the disease at the time of diagnosis. Despite showing improving PVR and compliance over the course of the study period, our two scleroderma patients had a deteriorating clinical course thereafter and ultimately died from PAH complications. The liver cirrhosis patient showed mild deterioration of his hemodynamics despite escalation of treatment and eventually passed. The HHT patient showed hemodynamic improvement and remained clinically stable. Out of all 14 patients, the MCTD patients were the ones with the greatest combined hemodynamic and clinical improvements, with an improvement from NYHA functional class IV to class II in one of the MCTD patients, in addition to the hemodynamic changes mentioned above.
Discussion
In the present study of 14 patients with various etiologies of PAH, the following important findings were observed: (I) the mean age was 52 years and all except one were female; (II) the RC constant remained stable over the mid-term follow-up; (III) patients with MCTD had more favorable hemodynamics, as evidenced by higher RC and PAC values at a given PVR, when compared with non-MCTD PAH; and, (IV) mean CO was decreased across PAH etiologies, and 50% of patients were in NYHA functional class III or IV. The findings in patients with MCTD are salient, as PAH is the primary cause of death in this population (7,8).
Arterial compliance as previously studied in the systemic circulation is directly related to the ratio of elastin to collagen, and has also been directly implicated in the compliance property of the pulmonary arteries (2,9,10). One proposed theory is that the pulmonary arteries in MCTD are richer in elastin than in other PAH etiologies, which may explain why those patients tend to respond better to treatment. At the molecular level, cytokines such as CCL-5 which mediate the production of endothelin, and thus indirectly promote vasoconstriction, were found to be upregulated in the lungs of PAH patients as compared to controls (11). In addition, growth factors such as platelet derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) promote angiogenesis and have been demonstrated to be over-expressed in patients with PAH (12,13). All the MCTD patients in the study were treated with endothelin receptor antagonists, which likely contributed to their overall superior pulmonary arterial compliance.
Prior reports have also suggested a beneficial effect of immunosuppressive therapy on PAH severity in MCTD (14). In addition to PDGF and VEGF, circulating interleukin (IL)-1, IL-6, and autoantibodies in MCTD also trigger pulmonary arterial vasoconstriction (15-17). Sanchez et al., tested monthly IV boluses of cyclophosphamide in 28 patients with various PAH etiologies. Three (38%) MCTD patients in the treatment arm improved both clinically and hemodynamically, suggesting a possible role of immunosuppressive therapy in improving arterial compliance. In our series, 2 out of the 3 MCTD patients were on immunosuppressive therapy for the duration of the study.
The primary strength of the current analysis is the detailed hemodynamic data gathered in the included cases, assessment of different etiologies of PAH, and mid-term follow-up. With this in mind, one must consider several limitations when interpreting the data. Firstly, the study was retrospective with a small sample size. This limits the statistical power for inter- and intragroup comparisons of baseline disease burden and treatment response. Secondly, a total of 68 patients were excluded, with the most common cause being incomplete right heart catheterization data, which represents a form of attrition bias. Thirdly, since the MCTD patients were on several therapies, including immunosuppressants, endothelin receptor inhibitors and phosphodiesterase-5 inhibitors, it precludes investigation into the distinct effects of each drug on the improved compliance and overall superior performance of MCTD patients. Finally, all but one patient was female which limits generalizability of the findings to male individuals. As such, the results are hypothesis-generating for larger prospective studies.
In conclusion, this small and mainly female cohort provides important insights into the hemodynamic and clinical characteristics of PAH. The relationship between PAC and PVR remains stable at mid-term follow-up, suggesting that compliance is an important disease marker past the early stages. MCTD patients had a more favorable hemodynamic profile than non-MCTD PAH patients. Given the high morbidity observed in this population, robust prospective studies to establish effective therapeutic regimens are paramount.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Christos G. Mihos) for the series “Novel Concepts in Cardiopulmonary and Structural Heart Disease” published in Journal of Thoracic Disease. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.02.20). The series “Novel Concepts in Cardiopulmonary and Structural Heart Disease” was commissioned by the editorial office without any funding or sponsorship. CGM served as the unpaid Guest Editor of the series and serves as an unpaid editorial member of Journal of Thoracic Disease from Jan 2019 to Dec 2020. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Mount Sinai Medical Center Institutional Review Board (No. FWA 00000176 IRB ID: 18-34-H-10).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dyer K, Lanning C, Das B, et al. Noninvasive Doppler tissue measurement of pulmonary artery compliance in children with pulmonary hypertension. J Am Soc Echocardiogr 2006;19:403-12. [Crossref] [PubMed]
- Cavalcante JL, Lima JA, Redheuil A, et al. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol 2011;57:1511-22. [Crossref] [PubMed]
- Mahapatra S, Nishimura RA, Oh JK, et al. The prognostic value of pulmonary vascular capacitance determined by Doppler echocardiography in patients with pulmonary arterial hypertension. J Am Soc Echocardiogr 2006;19:1045-50. [Crossref] [PubMed]
- Vonk Noordegraaf A, Westerhof BE, Westerhof N. The relationship between the right ventricle and its load in pulmonary hypertension. J Am Coll Cardiol 2017;69:236-43. [Crossref] [PubMed]
- Lankhaar JW, Westerhof N, Faes TJ, et al. Quantification of right ventricular afterload in patients with and without pulmonary hypertension. Am J Physiol Heart Circ Physiol 2006;291:H1731-7. [Crossref] [PubMed]
- Lankhaar JW, Westerhof N, Faes TJ, et al. Pulmonary vascular resistance and compliance stay inversely related during treatment of pulmonary hypertension. Eur Heart J 2008;29:1688-95. [Crossref] [PubMed]
- Yoshida S. Pulmonary arterial hypertension in connective tissue diseases. Allergol Int 2011;60:405-9. [Crossref] [PubMed]
- Mathai SC, Hassoun PM. Pulmonary arterial hypertension in connective tissue diseases. Heart Fail Clin 2012;8:413-25. [Crossref] [PubMed]
- Chemla D, Lau EM, Papelier Y, et al. Pulmonary vascular resistance and compliance relationship in pulmonary hypertension. Eur Respir J 2015;46:1178-89. [Crossref] [PubMed]
- Thenappan T, Prins KW, Pritzker MR, et al. The critical role of pulmonary arterial compliance in pulmonary hypertension. Ann Am Thorac Soc 2016;13:276-84. [PubMed]
- Dorfmüller P, Zarka V, Durand-Gasselin I, et al. Chemokine RANTES in severe pulmonary arterial hypertension. Am J Respir Crit Care Med 2002;165:534-9. [Crossref] [PubMed]
- Schermuly RT, Dony E, Ghofrani HA, et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest 2005;115:2811-21. [Crossref] [PubMed]
- Tuder RM, Chacon M, Alger L, et al. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J Pathol 2001;195:367-74. [Crossref] [PubMed]
- Sanchez O, Sitbon O, Jaïs X, et al. Immunosuppressive therapy in connective tissue diseases-associated pulmonary arterial hypertension. Chest 2006;130:182-9. [Crossref] [PubMed]
- Humbert M, Monti G, Brenot F, et al. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med 1995;151:1628-31. [Crossref] [PubMed]
- Hassoun PM, Mouthon L, Barberà JA, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol 2009;54:S10-9. [Crossref] [PubMed]
- Isern RA, Yaneva M, Weiner E, et al. Autoantibodies in patients with primary pulmonary hypertension: association with anti-Ku. Am J Med 1992;93:307-12. [Crossref] [PubMed]


