
Prognostic factors of recurrence of malignant pleural effusion: what is the role of neoplasia progression?
Introduction
Malignant pleural effusion (MPE) accounts for more than 125,000 hospital admissions per year in the USA alone (1), with an estimated inpatient cost >US$5 billion a year (1) and the incidence of MPE is still increasing, due to the continuous increase in new cancer diagnosis (2,3). The MPE-associated progressive dyspnea or cough is often debilitating and impairs quality of life (4), with severe respiratory limitation (5,6). It is known that MPE recurs rapidly, sometimes within a month after an initial thoracocentesis in a considerable number of patients (7,8). However, some patients do not have MPE recurrence. Events competing with MPE recurrence after the thoracocentesis are not rare, such as death from any other complications of the primary tumor or response after systemic treatment. Since MPE is associated with an average survival of 4–7 months (9), more accurate prediction of prognosis may help recognize patients at higher risk of pleural recurrence, aiming to individualize more intensive treatment strategies, such as pleurodesis or indwelling pleural catheter and, thus, minimize adversities in the end stages of the disease (10,11).
However, to date, there have been few studies that evaluated factors, including systemic therapy, associated with MPE recurrence (12-15). The aim of this study was to identify risk factors of recurrence in symptomatic patients only, who required a pleural approach.
Methods
A prospectively assembled database of cases with pleural effusion treated at a single institution, between April 01, 2016 to December 30, 2017, analyzed a subset of patients with symptomatic MPE. Patients younger than 18 years, patients with pleural empyema, chylothorax and patients with asymptomatic neoplastic pleural effusion were excluded. This study was approved by the Research Ethics Committee of the institution (protocol No. 49258615.4).
MPE was characterized as the presence of malignant cells in the pleural fluid, those with pleural infiltration recognized in the pathological evaluation or in patients with metastatic cancer in other sites, validated by the pathological analyses and pleural effusion with no additional diagnosed causes after review by the clinical team. Recurrence of pleural effusion was defined as the need for a new pleural procedure after the first pleural palliative procedure. The need for a new pleural procedure was evaluated monthly until one year after the inclusion of the last patient.
Preoperative data were collected to allow the investigation of prognostic factors, which may have an impact on recurrence. The obtained data included basic demographics, body mass index (BMI) on the day before the procedure, primary tumor site, American Society of Anesthesiologists (ASA) health status categorization, performance status according to the Eastern Cooperative Oncology Group (ECOG) rating, white blood cells (WBC), neutrophils and lymphocytes, neutrophil/lymphocyte ratio (NLR), red blood cells (RBC) and platelets/lymphocyte ratio. Metastatic sites were also evaluated, which was defined as presence of any numbers of metastasis at each organ.
Regarding the postoperative period, we analyzed pleural effusion recurrence, the palliative approach used, the volume of drained liquid, the presence of malignant cells in the pleural fluid, in addition to the biochemical profile comprehending adenosine deaminase (ADA), lymphocytes, total protein, lactate dehydrogenase (DHL), glucose and albumin in the pleural fluid, and pleural pH. We also evaluated the presence of diffuse pleural thickening and pulmonary infiltrate through chest computed tomography. These characteristics were described as present or absent and identified in the chest computed tomography reports prior to the palliative procedure. Systemic treatment was also evaluated. Patients were classified into three groups at MPE diagnosis: systemic treatment-naïve patients, patients who received first-line systemic treatment and patients receiving second-line systemic treatment or further therapy. Systemic treatment options were chosen at the discretion of the clinical oncology team and were not standardized.
Pleural procedure
Pleural approach was determined according to the multidisciplinary team’s decision and the guidelines of the British Thoracic Society (BTS) (10). Therapeutic pleural aspiration (TPA), defined as thoracocentesis for effusions that affected up to two-thirds of the hemithorax or small-bore chest tube for effusions that affected more than two-thirds of the hemithorax, was performed in patients that the multidisciplinary team considered as developing a pleural response to systemic treatment. TPA was also performed in patients with a life expectancy shorter than 30 days, according to the British Thoracic Society and the multidisciplinary team. The other patients were submitted to more aggressive procedures. High-risk patients for general anesthesia were submitted pleural drainage using a chest tube under local anesthesia. After full lung re-expansion and fluid drainage <200 milliliters/day, pleurodesis was performed through the drain. Moreover, an indwelling pleural drain was offered to patients considered unfit for general anesthesia as of December 2014.
Patients operated under total anesthesia were submitted to a video-assisted thoracoscopy (VAT). After suitable effusion samples were obtained for cytological analyses and cultures, several pleural fragments were obtained from any irregular areas; in the absence of irregular areas, a number of random areas were sampled. Frozen-section histopathological analysis was performed if there was no preoperative cancer diagnosis. If full lung re-expansion was attained, immediate pleurodesis was performed by insufflating 4 g of sterilized talc.
The chest tube was removed five days after the procedure in patients who did not attain full lung re-expansion. All patients were evaluated monthly to verify pleural recurrence, which was defined as need for a new approach (thoracocentesis or indwelling drain). In our institution, we wait five days to remove the drain, in an attempt to expand the lung with physical therapy and allow the pleurodesis with talc to be performed.
Statistical analysis
The descriptive analysis of data was performed through absolute and relative frequencies, measures of central tendency and dispersion.
In the analysis of recurrence-free survival, time, truncated at 12 months, was calculated between the date of surgery until the date of recurrence or last status of the patient. The quantitative variables without definite cutoff points were submitted to the ROC (receiver operating characteristic) curve, using a sub-sample of 50% of the recorded cases. Cutoff points were defined as the ones with sensitivity and specificity values >0.80. In the survival analysis, the Kaplan-Meier test was applied, and the log-rank test was used for the comparison between the curves. Univariate and multiple Cox regression models were used to evaluate the risk of recurrence (hazard ratio) and their respective 95% confidence intervals (95% CI). The variables that were tested in the multiple model were the ones with a P value <0.05 in the univariate analysis.
The level of statistical significance was set at 5%. The data were entered in Excel and analyzed using the SPSS program (Statistical Package for the Social Sciences), version 22.0 for Windows.
Results
Of the 288 included patients, 62.5% were females. The patients’ mean age was 59.8 years (SD =13.8), with a median of 61 years, ranging from 18 to 96 years of age. Regarding the nutritional status, 60.1% had normal weight at the time of recurrence diagnosis. The most frequent main procedure was pleurodesis (43.1%), followed by drainage (41.2%). ECOG performance status of the patients varied, with 41.7% presented with grade 2 (Table 1).
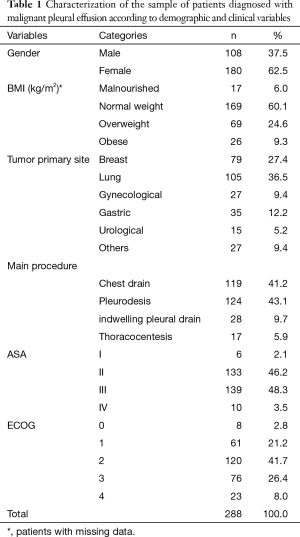
Full table
Disease recurrence occurred in 56 patients (19.4%). The mean time was 1.5 months (SD =3.2), with a median of 0.3 months (range, <0.1 to 19.7 months).
Recurrence-free survival was of 76.6% at 6 months and 73.3% at 12 months. Table 2 shows that patients submitted to the pleurodesis procedure had, in a 12-month period, a longer recurrence-free survival of 84.6%, with HR =0.33 (95% CI, 0.17–0.63) when compared to patients who underwent to the chest drain. On the other hand, patients who underwent thoracocentesis as the main procedure had, in a 6-month period, a longer recurrence-free survival of 35.3%, with HR =4.74 (95% CI, 2.40–9.36) of having recurrence when compared to the reference group.
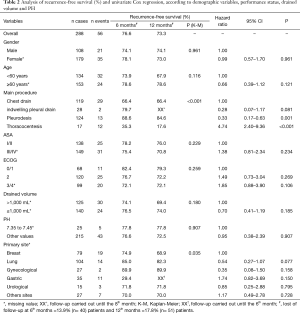
Full table
Patients with lymphocytes <2,900×10−9/L had longer recurrence-free survival than patients with ≥2,900 lymphocytes (79.1% versus 58.2%, P=0.008). The risk of a patient with ≥2,900 lymphocytes showing recurrence in up to 12 months is HR =2.02 (95% CI, 1.18–3.46) when compared to a patient with lymphocyte values <2,900×10−9/L. As for platelets, patients with values ≥150,000 had a recurrence-free survival of 76.8%, whereas patients with values <150,000 showed a percentage of recurrence-free survival of 42.0% (P=0.001), i.e., the risk of recurrence in patients with platelet values <150,000 mm/dL was HR =2.82 (95% CI, 1.48–5.38) (Table 3).
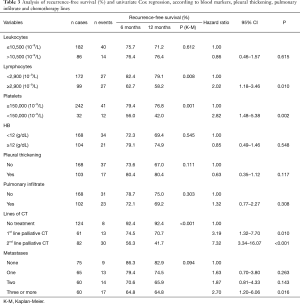
Full table
Regarding the chemotherapy (CT) lines of treatment, in a 12-month period, the percentage of recurrence-free survival for the untreated, those receiving first-palliative and second-palliative therapy categories were 92.4%, 70.7% and 41.7%, respectively (P<0.001). Cox univariate analysis showed that the risk of recurrence for those submitted to the 1st line of palliative CT was HR =3.19 (95% CI, 1.32–7.70) and for the 2nd line of palliative CT, HR =7.32 (95% CI, 3.34–16.07). Regarding the number of metastases, having 3 or more was shown to be a risk factor [HR =2.7 (95% CI, 1.2–6.06)] (Table 3). The variables in Table 4 did not show statistical significance between the survival rates.
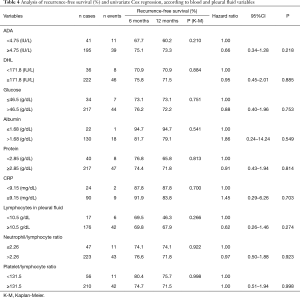
Full table
The independent factors for recurrence-free survival were procedure and chemotherapy lines. Patients who were submitted to pleurodesis had a protective factor for recurrence, with an HR =0.34 (95% CI, 0.15–0.74, P=0.007). On the other hand, patients submitted to the 1st and 2nd line of palliative CT had, respectively, an HR risk =2.81 (95% CI, 1.10–7.28, P=0.034) and HR =3.23 (95% CI, 1.33–7.84, P=0.010). These were adjusted for the variables age and lymphocyte in the fluid (Table 5, Figure 1).
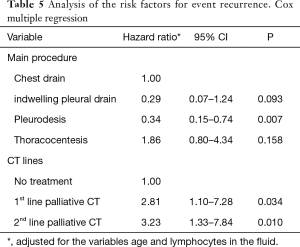
Full table
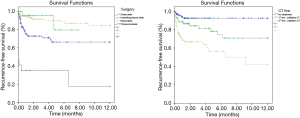
Discussion
In this single-center cohort study that included 288 patients with MPE, we observed that the patient’s systemic treatment phase is associated with an increase in the risk of MPE, with an HR =2.8 (95% CI, 1.10–7.28, P=0.034) for first-line systemic treatment and HR =3.23 (95% CI, 1.33–7.84, P=0.01) for second-line systemic treatment, in comparison with treatment-naïve patients. We also reported that pleurodesis significantly decreased the risk of pleural malignant recurrence (HR =0.34, 95% CI, 0.15–0.74, P=0.007). The most important finding of our study is the association between pleural malignant effusion recurrence and the patients’ disease progression status, characterized by the patient’s treatment stage. Patients submitted to pleural effusion at the time of diagnosis, i.e., systemic treatment-naïve ones, were less likely to have effusion recurrence than patients who had neoplastic pleural effusion after the administration of systemic treatment. Additionally, patients who were already receiving the 2nd-line systemic treatment had a higher risk of recurrence than patients who were receiving the 1st-line systemic treatment. These results suggest that patients at advanced stages of metastatic neoplasia have a higher risk of recurrence regardless of the proposed pleural treatment.
Identifying factors associated to MPE recurrence at the time of diagnosis is important, as it can individualize the approach, significantly preventing the impact on patient quality of life. Patients with high risk of recurrence can be submitted to a definitive treatment for pleural malignant effusion as the first option. However, there are several articles evaluating factors associated with mortality (16-18) and few studies reporting prognostic factors associated with pleural effusion recurrence (19-24). Moreover, most studies only analyzed pleurodesis agents as predictors of recurrence (20-23), and these studies were not designed to evaluate prognostic factors.
According to a recent meta-analysis, talc was compared with other sclerosing agents, indwelling pleural catheters, thoracoscopic mechanical pleurodesis and drainage alone. The success rate of talc pleurodesis was significantly higher than that of other therapies (relative risk, 1.21; 95% CI, 1.01–1.45; P=0.035) (25). Two randomized trials recently analyzed MPE recurrence. The TIME 1 randomized trial reported that pleurodesis using a 12-French chest tube is more associated with higher MPE recurrence after 3 months than a 24-French chest tube (30% vs. 24% difference, −6%; 1-sided 95% CI, −20% to ∞; P=0.14 for noninferiority) (26). The TIME 2 randomized trial reported MPE recurrence comparing indwelling pleural catheter versus pleurodesis. This study reported lower risk of MPE recurrence with indwelling pleural catheter compared with talc pleurodesis (odds ratio =0.21; 95% CI, 0.04–0.86; P=0.03) (27). The association between biomarkers and MPE recurrence was also studied (28,29). The report of Hsu et al. compared pleural fluid concentrations of three biomarkers between patients who had MPE recurrence and patients who reached successful pleurodesis. The mean values were not significant between both groups: osteopontin 809.53±287.72 vs. 361.54±71.80 ng/mL; P=0.151, vascular endothelial growth factor (VEGF) 5,610.94±2,040.61 vs. 3,564.96±1,044.12 pg/mL; P=0.383 and urokinase-type plasminogen activator 99.04±53.88 vs. 25.80±3.22 ng/mL; P=0.198 (28). There are also few studies evaluating the association between systemic treatment and MPE. Tamiya et al. reported another phase-II study including 23 patients with bevacizumab and carboplatin – paclitaxel. The MPE control rate showed a non-significant improvement with the combination of CP with Bev (CP, 78.3%; CP with Bev, 91.3%; P=0.08) and the median pleural progression-free survival was 8.8 months (95% CI, 6.7–13.8 months) (14,24). Usui et al., in a phase-II study that included 30 patients with MPE treated with a bevacizumab and carboplatin pemetrexed combination, reported a recurrence rate of 21.4% at the end of the study, with a median follow-up of 384 days (15). Regarding targeted therapy, Lin et al. reported the first-line treatment with tyrosine-kinase inhibitors in patients with MPE and non-small cell lung cancer, showing 43.4% of MPE recurrence during a median follow-up period of 1,050 days (23). Our study did not evaluate any specific treatment, but the association between the systemic treatment phase in which the patient was and MPE recurrence. To the best of our knowledge, this is the first study that demonstrated this association. Our previous study, including non-small cell lung cancer patients and MPE only found an association between patients at the second-line chemotherapy phase and MPE recurrence in the univariable analysis.
Our study showed that thoracentesis was associated with increased risk of pleural effusion recurrence, reinforcing the recommendations of the most recent guidelines. On the other hand, these guidelines do not take into account prognostic factors of recurrence. The BTS, ATS, STS and STR guidelines, as well as some reviews (10,30-32) recommend therapeutic thoracocentesis as the first approach to MPE. The aim would be to assess dyspnea relief. However, we know that these patients are receiving palliative treatment and, therefore, the fewer the procedures, the less the psychological and physical stress. Furthermore, we observe in clinical practice that virtually all symptomatic patients have some degree of symptom improvement, even when there is another associated disease, such as pulmonary embolism or lymphangitis. That occurs because the dyspnea reflects reduced chest wall compliance, depression of the ipsilateral diaphragm, mediastinal shift and lung volume reduction (33), including patients with trapped lung. Therefore, it would be reasonable to evaluate prognostic factors together with the recommended guidelines and, therefore, perhaps we could avoid thoracentesis as the initial procedure when managing symptomatic patients with MPE receiving the first or second line of treatment, since these patients would be at greater risk of recurrence, as shown in this study. Hence, we recommend a definitive treatment, such as pleurodesis, or long-term pleural catheter.
Our study has some limitations. The assessed population was referred to the thoracic surgery unit of a single hospital. Another important point is that we did not evaluate the effect of specific systemic therapies, such as targeted therapy for the treatment of MPE. We also included patients with several different types of cancer, which possibly influenced the pleural recurrence free-survival. The latter might decrease the accuracy of our recurrence risk measures.
We conclude that the patient’s systemic treatment phase for neoplasia is independently associated with the risk of recurrence for MPE after a palliative pleural approach. That is, patients receiving the first or second line of systemic treatment have a higher risk of MPE recurrence when compared to patients who underwent MPE treatment before starting the systemic treatment. The definitive treatment of MPE, such as pleurodesis, was associated with a lower risk of MPE recurrence.
Acknowledgments
We thank Librarian Denise Vieira Camacho and Stela Verzinhasse Peres for technical help.
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Research Ethics Committee of the institution (protocol No. 49258615.4). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Taghizadeh N, Fortin M, Tremblay A. US hospitalizations for malignant pleural effusions: data from the 2012 national inpatient sample. Chest 2017;151:845-54. [Crossref] [PubMed]
- American Thoracic Society. Management of malignant pleural effusions. Am J Respir Crit Care Med 2000;162:1987-2001. [Crossref] [PubMed]
- Cancer Statistics Registrations, England (Series MB1): Office of National Statistics, Stationary Office, 2010.
- Thomas R, Jenkins S, Eastwood PR, et al. Physiology of breathlessness associated with pleural effusions. Curr Opin Pulm Med 2015;21:338-45. [Crossref] [PubMed]
- Antunes G, Neville E, Duffy J, et al. BTS guidelines for the management of malignant pleural effusions. Thorax 2003;58:ii29-38. [Crossref] [PubMed]
- Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J 2001;18:402-19. [Crossref] [PubMed]
- Heffner JE, Klein JS. Recent advances in the diagnosis and management of malignant pleural effusions. Mayo Clin Proc 2008;83:235-50. [Crossref] [PubMed]
- Shaw PH, Agarwal R. Pleurodesis for malignant pleural effusions. Cochrane Database Syst Rev 2013;11:CD002916. [PubMed]
- Porcel JM, Gasol A, Bielsa S, et al. Clinical features and survival of lung cancer patients with pleural effusions. Respirology 2015;20:654-9. [Crossref] [PubMed]
- Roberts ME, Neville E, Berrisford RG, et al. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii32-40. [Crossref] [PubMed]
- Tan C, Sedrakyan A, Browne J, et al. The evidence on the effectiveness of management for malignant pleural effusion: a systematic review. Eur J Cardiothorac Surg 2006;29:829-38. [Crossref] [PubMed]
- Abrao FC, de Abreu IRLB, Viana GG, et al. Wet M1a non-small cell lung cancer: is it possible to predict recurrence of pleural effusion? J Thorac Dis 2018;10:808-15. [Crossref] [PubMed]
- Verma A, Chopra A, Lee YW, et al. Can EGFR-Tyrosine Kinase Inhibitors (TKI) Alone Without Talc Pleurodesis Prevent Recurrence of Malignant Pleural Effusion (MPE) in Lung Adenocarcinoma. Curr Drug Discov Technol 2016;13:68-76. [Crossref] [PubMed]
- Tamiya M, Tamiya A, Yamadori T, et al. Phase 2 study of bevacizumab with carboplatin-paclitaxel for non-small cell lung cancer with malignant pleural effusion. Med Oncol 2013;30:676-82. [Crossref] [PubMed]
- Usui K, Sugawara S, Nishitsuji M, et al. A phase II study of bevacizumab with carboplatin-pemetrexed in nonsquamous non-small cell lung carcinoma patients with malignant pleural effusions: North East Japan Study Group Trial NEJ013A. Lung Cancer 2016;99:131-36. [Crossref] [PubMed]
- Clive AO, Kahan BC, Hooper CE, et al. Predicting survival in malignant pleural effusion: development and validation of the LENT prognostic score. Thorax 2014;69:1098-104. [Crossref] [PubMed]
- Abrao FC, Peixoto RD, Abreu IR, et al. Prognostic factors in patients with malignant pleural effusion: Is it possible to predict mortality in patients with good performance status? J Surg Oncol 2016;113:570-4. [Crossref] [PubMed]
- Lim JU, Yeo CD, Kang HS, et al. Elevated pretreatment platelet-to-lymphocyte ratio is associated with poor survival in stage IV non-small cell lung cancer with malignant pleural effusion. Sci Rep 2019;9:4721. [Crossref] [PubMed]
- Das SK, Saha SK, Das A, et al. A study of comparison of efficacy and safety of talc and povidone iodine for pleurodesis of malignant pleural effusions. J Indian Med Assoc 2008;106:589-590, 592. [PubMed]
- Mohsen TA, Zeid AA, Meshref M, et al. Local iodine pleurodesis versus thoracoscopic talc insufflation in recurrent malignant pleural effusion: a prospective randomized control trial. Eur J Cardiothorac Surg 2011;40:282-6. [PubMed]
- Demmy TL, Gu L, Burkhalter JE, et al. Optimal management of malignant pleural effusions (results of CALGB 30102). J Natl Compr Canc Netw 2012;10:975-82. [Crossref] [PubMed]
- Stefani A, Natali P, Casali C, et al. Talc poudrage versus talc slurry in the treatment of malignant pleural effusion. A prospective comparative study. Eur J Cardiothorac Surg 2006;30:827-32. [Crossref] [PubMed]
- Lin JB, Lai FC, Li X, et al. Sequential treatment strategy for malignant pleural effusion in non-small cell lung cancer with the activated epithelial grow factor receptor mutation. J Drug Target 2017;25:119-24. [Crossref] [PubMed]
- Tamiya M, Tamiya A, Yasue T, et al. Vascular endothelial growth factor in plasma and pleural effusion is a biomarker for outcome after bevacizumab plus carboplatin-paclitaxel treatment for non-small cell lung cancer with malignant pleural effusion. Anticancer Res 2016;36:2939-44. [PubMed]
- Xia H, Wang XJ, Zhou Q, et al. Efficacy and Safety of Talc Pleurodesis for Malignant Pleural Effusion: A Meta-Analysis. PLoS One 2014;9:e87060. [Crossref] [PubMed]
- Rahman NM, Pepperell J, Rehal S, et al. Effect of opioids vs NSAIDs and larger vs smaller chest tube size on pain control and pleurodesis efficacy among patients with malignant pleural effusion: The TIME1 Randomized Clinical Trial. JAMA 2015;314:2641-53. [Crossref] [PubMed]
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012;307:2383-9. [Crossref] [PubMed]
- Hsu LH, Hsu PC, Liao TL, et al. Pleural fluid osteopontin, vascular endothelial growth factor, and urokinase-type plasminogen activator levels as predictors of pleurodesis outcome and prognosticators in patients with malignant pleural effusion: a prospective cohort study. BMC Cancer 2016;16:463. [Crossref] [PubMed]
- Pantazopoulos I, Xanthos T, Vlachos I, et al. Pleural fluid glucose: A predictor of unsuccessful pleurodesis in a preselected cohort of patients with malignant pleural effusion. J BUON 2014;19:1018-23. [PubMed]
- Nam HS, Ryu JS. Diagnosis and management of malignant pleural effusion. Korean J Med 2011;81:167-73.
- Zarogoulidis K, Zarogoulidis P, Darwiche K, et al. Malignant pleural effusion and algorithm management. J Thorac Dis 2013;5 Suppl 4:S413-9. [PubMed]
- Feller-Kopman DJ, Reddy CB, DeCamp MM, et al. Management of Malignant Pleural Effusions. An Official ATS/STS/STR Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198:839-49. [Crossref] [PubMed]
- Judson M, Sahn S. Pulmonary physiologic abnormalities caused by pleural disease. Semin Respir Crit Care Med 1995;16:346-53. [Crossref]


