Impact of metformin on survival outcome of esophageal squamous cell carcinomas patients undergoing surgical resection: a multicenter retrospective study
Introduction
For malignant tumors, diabetes is not only one of the causes of morbidity, but also one of the risk factors leading to poor survival (1-4). The effect of diabetes on the survival of esophageal cancer is currently controversial. The effect of diabetes on the survival of patients with esophageal cancer has been studied by many scholars. Most studies confirm that diabetes mellitus is associated with a worse survival (4), however, some studies suggest that diabetes is not an independent risk factor for survival (5,6).
The treatment of type2 diabetes mellitus (T2DM), especially metformin, has been proved by numerous studies in recent years to improve the survival of patients with malignant tumors (7-10). For patients with esophageal squamous cell carcinoma (ESCC), many molecular mechanisms have been proved that metformin can inhibit tumor progression (11-13). However, the inhibitory effect of metformin on ESCC lacks direct follow-up observation with big data. Another point to ponder is whether metformin has a consistent tumor-suppressing effect in all patients with ESCC.
In this study, we first used a large sample to re-examine the effect of type 2 diabetes on ESCC survival outcomes. Since metformin is believed to improve the survival of some cancer patients, we conducted subgroup analysis to further investigate whether the relationship between T2DM and ESCC outcome is related to metformin.
Methods
Patients
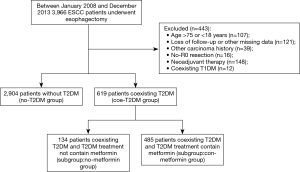
The institutional review board of five hospitals involved in this study approved this study. All subjects involved in the study provided informed consent. Briefly, we collected patients with newly diagnosed ESCC pathologically from 2008 to 2013.All patients underwent surgical resection. Basic information (including age, sex, smoking status, alcohol consumption, BMI) was collected by consulting inpatient medical records. Phone or email to collect follow-up data. The follow-up data were completed with the assistance of the household registration department and hospitals. The data collection route is shown in Figure 1.
Main observation indicators
The most important outcome measure is overall survival (OS), defined as from the date of surgery to the date of death or the last known survival date. The second major outcome measure was disease-free survival (DFS), defined as the time between the date of surgery and the recurrence of cancer.
Statistical analysis method
Data were compared across subgroups using OS and DFS, Informed consent Associations between T2DM and outcomes were estimated using the method of Kaplan-Meier to generate survival curves and assessed using the log-rank tests. Cox proportional hazards models were used as primary analyses, adjusting for age, gender, stage, performance status, smoking status and drinking status, and BMI. The same method was used to evaluate the associations between metformin use and outcomes for patients ESCC with T2DM. Factorial design was using to evaluate whether two factors interact. All reported P values are from two-sided tests. P value less than 0.05 was considered statistically significant. All statistical analyses used SPSS software version 20.0.
Results
Basic information of patients
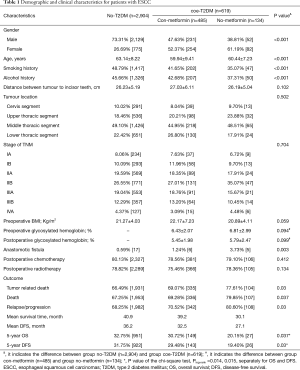
Finally, 3,523 ESCC patients were included in this study, and 2,432 patients relapsed and 2,396 died within 5 years after surgery. The mean follow-up time for these patients was 39.2 months (1.9–72.0 months). The 5-year OS and DFS of those patients included in this study were 31.99% and 30.97%, respectively. Gender, smoking status and drinking status were significantly different between ESCC with T2DM and without T2DM. For ESCC with T2DM patients, divided into two subgroups by the absence versus presence of metformin use. Gender, smoking status, drinking status, and TNM stage were no significantly different between these two subgroups. General information and clinical treatment of all patients were shown in Table 1.
Full table
Relationship between T2DM and OS and DFS
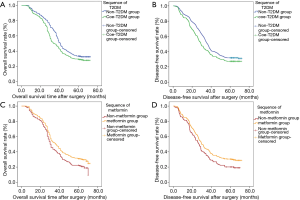
First, Kaplan-Meier curves were drawn, and the results showed that T2DM was significantly correlated with OS deterioration (log-rank test, P<0.001; Figure 2A). The 5-year OS rates of patients with T2DM was significantly lower than that of patients without T2DM (28.43% vs. 32.75%, log-rank test, P<0.001).
Univariate analysis showed that with T2DM was associated with worse OS after surgery for ESCC patients (HR =1.24; 95% CI, 1.12–1.38; P<0.001). In the multivariate Cox proportional hazard model adjusting for gender, smoking status and drinking status, the adjusted hazard ratio (HR adj) for T2DM was 1.19 (95% CI, 1.10–1.29; P<0.001) when compared with non-T2DM (Table 2).

Full table
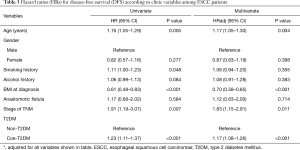
The same statistical method was used to analyze the effect of T2DM on DFS, and the results was similar to the results of OS, and that T2DM was significantly correlated with DFS deterioration (log-rank test, P<0.001; Figure 2B) (P<0.001). The 5-year DFS rates in patients with T2DM was significantly lower than those without T2DM (27.30% vs. 31.75%, log-rank test, P=0.03). Univariate analysis shown that T2DM was significantly associated with worse DFS (HR =1.23; 95% CI, 1.11–1.37; P<0.001). After a similar multivariate adjustment, HR adj for T2DM was 1.17 (95% CI, 1.08–1.26; P<0.001) when compared with non-T2DM (Table 3).
Full table
Relationship between metformin and OS and DFS for ESCC with T2DM patients
In stratified analyses, Kaplan-Meier curves showed that metformin use were significantly associated with better OS (Log-rank test, P=0.014; Figure 2C) and DFS (Log-rank test, P=0.015; Figure 2D). The 5-year OS rates in patients with metformin use (30.72%) was significantly higher than that without metformin use (20.15%) (P=0.014), and the 5-year DFS rates in patients with metformin use (29.48%) was significantly higher than those without metformin use (19.40%) (P=0.015).
In the univariate analysis, metformin use was significantly associated with better OS (HR =0.76; 95% CI, 0.61–0.95; P=0.015) and DFS (HR =0.76; 95% CI, 0.62–0.95; P=0.015). In the multivariate Cox proportional hazard model adjusting for clinical variables, HRadj for metformin use was 0.89 (95% CI, 0.80–0.99; P=0.031) for OS and 0.90 (95% CI, 0.83–0.98; P=0.013) for DFS relative to the absence of metformin use (Tables 4,5).

Full table
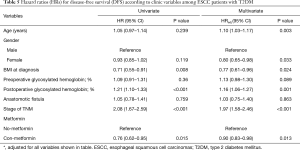
Full table
We further compared 5-year survival of group no-T2DM and subgroup coe-metformin, univariate COX regression indicated that there was difference in survival between the two groups (OS: HR =0.85; 95% CI, 0.75–0.95, P=0.004; DFS: HR =0.86; 95% CI, 0.77–0.97, P=0.010), and K-M curve showed that the survival benefit brought by metformin could not offset the survival harm brought by T2DM (Figure 3).

Discussion
Because of the complex pathogenesis and early symptoms are atypical of ESCC, most patients are in the progressive stage at the time of diagnosis (14). Although the treatment has been improving, including surgery and comprehensive treatment, the prognosis is still very unsatisfactory (15).
In recent years, many studies have found that the drugs used for some chronic diseases may affect the prognosis of tumors (16). Diabetes is considered to be one of the causes of esophageal cancer (5). Meanwhile, as one of the conventional drugs for diabetes treatment, metformin has been proved to improve the prognosis of patients by improving the pathological remission rate of neoadjuvant therapy for esophageal cancer (17).
Metformin is one of the most widely prescribed glucose-lowering agents for treatment of type 2 diabetes mellitus (T2DM) due to its superior safety profile and few side effects such as lactic acidosis and hypoglycemia (18). It has the dual effect of reducing the body weight and blood glucose of obese patients (19), making it one of the preferred drugs for obese T2DM patients. However, both obesity and diabetes are risk factors for tumorigenesis (1,5,20,21), does metformin reduce tumor incidence by reducing the patient’s weight or by controlling the patient’s diabetes? It has not been confirmed. Previous studies have elucidated this mechanism from a molecular biological perspective (10,13), but there is a lack of large sample multicentric clinical observations.
In previous clinical studies, the effect of metformin on the prognosis of esophageal cancer is controversial. Some studies have shown that metformin does not improve the prognosis of patients with esophageal cancer and may even weaken the efficacy of chemotherapy drugs (22,23). However, Sekino (12) prompt that metformin showed antitumor effects by inhibiting cell proliferation, tumor growth and Epithelial-mesenchymal transition (EMT) and inducing apoptosis in ESCC cell lines and xenograft models. These effects may have been induced by inhibiting NF-kB activation on ESCC. In addition to, Damelin et al. (23,24) show that the copper-bis (thiosemicarbazones), Cu-ATSM and Cu-GTSM, which are trapped in cells under reducing conditions, cause significant ESCC cytotoxicity, both alone and in combination with metformin.
The continuous development of molecular biology research provides impetus and theoretical support for us to carry out this multi-center and large-sample retrospective study.
Based on these previous studies, our study first confirmed that T2DM is indeed an independent risk factor for the prognosis of patients with ESCC through large sample data, and the combination of T2DM will indeed bring a worse prognosis, which is consistent with previous studies (1,5,25). In the stratified analysis, metformin was found to provide significant survival benefits for ESCC with T2DM patients. Of course, by looking at the K-M curve, we also found that metformin improved the prognosis of patients withT2DM with ESCC, this improvement did not seem to offset the risk of T2DM itself (Figure 3A,B).
Limitations
As a retrospective study, this paper has insuperable limitations. First, we missed the data of postoperative BMI changes of ESCC patients. The BMI mentioned in this study is patient’s BMI at the time of diagnosis. Most patients with esophageal cancer will lose weight after surgery (26), and metformin will also reduce the weight of diabetic patients, which will directly affect the recurrence of tumor. Therefore, the relationship between ESCC, metformin and weight change are worth further study. Secondly, fasting and postprandial blood glucose monitoring are the direct methods to compare blood glucose control in patients with diabetes. However, such data are missing in our study. HbA1C can only reflect the overall control of blood glucose in recent months, and cannot accurately reflect the fluctuation of blood glucose. Does metformin bring better prognosis to patients because of better blood glucose control in patients with esophageal cancer after surgery (1)? Thirdly, how does the endocrinologist decide whether to use metformin in the treatment of diabetes, and will these conditions affect the prognosis of patients? Fourthly, the anticancer effect of metformin may be related to the synergistic effect of other drugs, which has been confirmed by other studies (23,27,28). However, our retrospective study lacks the use record of other drugs when taking metformin. Finally, the biggest doubt for ours, whether ESCC without T2DM patients can benefit from metformin? Our team is applying to the ethics committee to conduct a multi-center, large-sample, prospective, randomized clinical study to address the above questions.
Conclusions
Coexisting T2DM is associated with worse survival outcomes in ESCC patients, and metformin may improve the prognosis of these patients.
Acknowledgments
We thank patients for their participation in this study. We also thank the team of TWM for data management and assistance to this project, and thank my good friends Hong Liu (MD, PHD) and Jiaxi He (MD, PHD) gave me guidance in writing the article.
Funding: None.
Footnote
Conflicts of Interest: JH serves as the unpaid Executive Editor-in-Chief of Journal of Thoracic Disease. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the ethics committee of the first affiliated hospital of Guangzhou medical university, and ethics committee of all participating institutions agree to implement.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Okamura A, Watanabe M, Imamura Y, et al. Glycemic Status and Prognosis of Patients with Squamous Cell Carcinoma of the Esophagus. World J Surg 2017;41:2591-7. [Crossref] [PubMed]
- Yoshida Y, Schmaltz CL, Jackson-Thompson J, et al. The effect of metabolic risk factors on cancer mortality among blacks and whites. Transl Cancer Res 2019;8:S389-96. [Crossref]
- Hjartåker A, Langseth H, Weiderpass E. Obesity and diabetes epidemics: cancer repercussions. Adv Exp Med Biol 2008;630:72-93. [Crossref] [PubMed]
- Breyer J. Does diabetes mellitus play an independent prognostic role in kidney cancer? Ann Transl Med 2019;7:S382. [Crossref] [PubMed]
- Yao W, Meng Y, Lu M, et al. Impact of type 2 diabetes mellitus on short-term and long-term outcomes of patients with esophageal squamous cell cancer undergoing resection: a propensity score analysis. Cancer Commun (Lond) 2018;38:14. [Crossref] [PubMed]
- Hayashi Y, Correa AM, Hofstetter WL, et al. Patients with high body mass index tend to have lower stage of esophageal carcinoma at diagnosis. Dis Esophagus 2012;25:614-22. [Crossref] [PubMed]
- Kim HJ, Lee S, Chun KH, et al. Metformin reduces the risk of cancer in patients with type 2 diabetes: An analysis based on the Korean National Diabetes Program Cohort. Medicine (Baltimore) 2018;97:e0036. [Crossref] [PubMed]
- Bowker SL, Majumdar SR, Veugelers P, et al. Increased cancer related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 2006;29:254-8. [Crossref] [PubMed]
- Libby G, Donnelly LA, Donnan PT, et al. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 2009;32:1620-5. [Crossref] [PubMed]
- Feng Y, Ke C, Tang Q, et al. Metformin promotes autophagy and apoptosis in esophageal squamous cell carcinoma by downregulating Stat3 signaling. Cell Death Dis 2014;5:e1088. [Crossref] [PubMed]
- Yu H, Bian X, Gu D, et al. Metformin Synergistically Enhances Cisplatin-Induced Cytotoxicity in Esophageal Squamous Cancer Cells under Glucose-Deprivation Conditions. Biomed Res Int 2016;2016:8678634.
- Sekino N, Kano M, Matsumoto Y, et al. Antitumor effects of metformin are a result of inhibiting nuclear factor kappa B nuclear translocation in esophageal squamous cell carcinoma. Cancer Sci 2018;109:1066-74. [Crossref] [PubMed]
- Nakayama A, Ninomiya I, Harada S, et al. Metformin inhibits the radiation-induced invasive phenotype of esophageal squamous cell carcinoma. Int J Oncol 2016;49:1890-8. [Crossref] [PubMed]
- Newton AD, Predina JD, Xia L, et al. Surgical Management of Early-Stage Esophageal Adenocarcinoma Based on Lymph Node Metastasis Risk. Ann Surg Oncol 2018;25:318-25. [Crossref] [PubMed]
- Li L, Zhao L, Lin B, et al. Adjuvant Therapeutic Modalities Following Three-field Lymph Node Dissection for Stage II/III Esophageal Squamous Cell Carcinoma. J Cancer 2017;8:2051-9. [Crossref] [PubMed]
- Joo MK, Park JJ, Chun HJ. Additional Benefits of Routine Drugs on Gastrointestinal Cancer: Statins, Metformin, and Proton Pump Inhibitors. Dig Dis 2018;36:1-14. [Crossref] [PubMed]
- Van De Voorde L, Janssen L, Larue R, et al. Can metformin improve 'the tomorrow' of patients treated for oesophageal cancer? Eur J Surg Oncol 2015;41:1333-9. [Crossref] [PubMed]
- Campbell RK, White JR Jr, Saulie BA. Metformin: a new oral biguanide. Clin Ther 1996;18:360-71; discussion 359. [Crossref] [PubMed]
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: aconsensus algorithm for the initiation and adjustment of therapy: aconsensus statement of the American Diabetes Association and theEuropean Association for the Study of Diabetes. Diabetes Care 2009;32:193-203. [Crossref] [PubMed]
- Zheng J, Zhao M, Li J, et al. Obesity-associated digestive cancers: A review of mechanisms and interventions. Tumour Biol 2017;39:1010428317695020. [Crossref] [PubMed]
- Jiang X, Bernstein L, Tseng CC, Wu AH. Diabetes and risk of esophageal and gastric adenocarcinomas. Int J Cancer 2012;131:1417-22. [Crossref] [PubMed]
- Spierings LE, Lagarde SM, van Oijen MG, et al. Metformin Use During Treatment of Potentially Curable Esophageal Cancer Patients is not Associated with Better Outcomes. Ann Surg Oncol 2015;22 Suppl 3:S766-71. [Crossref] [PubMed]
- Damelin LH, Jivan R, Veale RB, et al. Metformin induces an intracellular reductive state that protects oesophageal squamous cell carcinoma cells against cisplatin but not copper-bis(thiosemicarbazones). BMC Cancer 2014;14:314. [Crossref] [PubMed]
- Jivan R, Peres J, Damelin LH, et al. Disulfiram with or without metformin inhibits oesophageal squamous cell carcinoma in vivo. Cancer Lett 2018;417:1-10. [Crossref] [PubMed]
- Twarock S, Reichert C, Peters U, et al. Hyperglycaemia and aberrated insulin signalling stimulate tumour progression via induction of the extracellular matrix component hyaluronan. Int J Cancer 2017;141:791-804. [Crossref] [PubMed]
- Liu B, Cheng B, Wang C, et al. The prognostic significance of metabolic syndrome and weight loss in esophageal squamous cell carcinoma. Sci Rep 2018;8:10101. [Crossref] [PubMed]
- Lee J, Yesilkanal AE, Wynne JP, et al. Effective breast cancer combination therapy targeting BACH1 and mitochondrial metabolism. Nature 2019;568:254-8. [Crossref] [PubMed]
- Dowling RJ, Lam S, Bassi C, et al. Metformin Pharmacokinetics in Mouse Tumors: Implications for Human Therapy. Cell Metab 2016;23:567-8. [Crossref] [PubMed]