Clinical and economic comparative effectiveness of robotic-assisted, video-assisted thoracoscopic, and open lobectomy
Introduction
Over the past decades, there has been steady and significant adoption of minimally invasive video-assisted thoracoscopic lobectomy (VL) (1). Compared with open lobectomy (OL), VL has been associated with improvements in the reduction of complications, length of stay (LOS), costs, and has similar or slightly better oncologic outcomes than OL (1-3). Despite these published advantages, VL has not become the preferred technique for the majority of lobectomies.
Robotic-assisted lobectomy (RL) provides an alternative, minimally invasive technique for lobectomy. Perceived advantages include 3D high-definition video, improved stability, and wristed instruments for improved dexterity. Early clinical experience reporting safety and feasibility outcomes (4) has led to significant interest, but also raises questions regarding the learning curve, costs, and perioperative outcomes (5). Since it has been nearly a decade since the initial reports of RL and its subsequent routine adoption at many centers across the US, it is timely and necessary to analyze the nationwide trends in adoption, clinical outcomes, and cost of this procedure. There have been several publications assessing clinical outcomes of RL at various time points in different databases but do not include the same time periods or examine costs and the effects of volume and time period on outcomes (6-10).
The objectives of this study were to assess trends in the utilization of OL, VL, and RL to treat patients with lung malignancy; assess the perioperative outcomes between the approaches; and analyze the costs of RL compared with those of VL and OL.
Methods
Patient data from the Premier Healthcare Database, an all payer database which includes an excess of 700 community and academic hospitals in the United States and approximately 20% of inpatient visits, was used for this retrospective study. The database captures patient, surgeon, and hospital characteristics, as well as 30-day outcomes and direct and indirect costs. As the database contains aggregated, de-identified patient information and is compliant with the Health Insurance Portability and Accountability Act, the study did not require approval from an institutional review board.
Adults (≥18 years) who had a lobectomy for neoplasm from January 1, 2008, through September 30, 2015, were included. The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) was used to define cases and approaches (Table S1). Minimally invasive lobectomies converted to OL were classified by the initially intended approach (VL or RL). Exclusion criteria included patients aged <18 years, prior thoracotomy, emergent or urgent cases, operating room (OR) duration ≤1 or ≥24 hours, LOS ≤0 days, and total in-hospital cost ≤$0. Cases with missing data were excluded.

Full table
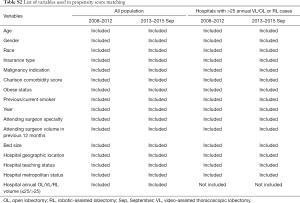
Analyzed data included patient demographics (age, sex, race/ethnicity, insurance type) and clinical characteristics (Charlson Comorbidity Score, obesity, smoking status, indication/diagnosis for lobectomy). Hospitals were classified by teaching status, bed size, urban/rural area, and census region. Surgeon specialty was provided by the database and classified as thoracic, cardiovascular, or other. Surgeon volume was determined individually and calculated as the number of lobectomies at a given hospital in the 12-month period before the date of surgery. Hospital annual case volume was calculated as the annual number of lobectomies in the hospital by surgical approaches in each calendar year. Intra- and postoperative complications, blood transfusion, conversion rate, and resource utilization including OR, intensive care unit (ICU) duration, and LOS during hospitalization were also examined. In-hospital and perioperative 30-day costs were calculated by hospital-reported total costs including fixed (overhead) and variable (direct) costs. The capital cost of laparoscopic/robotic equipment is included in the total and indirect costs. All costs were inflation-adjusted to 2015 US dollars using the historical US consumer price index (CPI).
As a means to determine the effect of the time period on outcomes, the study period was divided into an early and late period. The early period was defined as January 2008 through December 2012 and the late period included January 2013 through September 2015. Due to changes in the ICD-9 codes to ICD-10 in October 2015, the end of the study period was chosen as September 2015. The time periods were based on when the adoption rate exceeded 10% for RL as a transition from early exploration to more common adoption. A previous publication by Kent et al. (11) that analyzed the State Inpatient Databases for trends and clinical outcomes of RL from 2008–2010 that showed a very early adoption pattern of 3.4%, and a publication by Oh et al. that analyzed the Premier Database for trends and clinical outcomes of RL from 2011–2015 that showed that after 2012 the adoption rate exceeded 10% for the first time (9). Based on this information, we considered the early period [2008–2012] as a time of early adopters going through their collective learning curve and the late period (2013–Sept 2015) as a time when RL had become more widespread (>10% adoption). No previous study has analyzed RL in the Premier Database over nearly 8 years from 2008–September 2015, which was the focus of the present study.
A subgroup analysis focused on annual hospital volume >25 RL or VL cases to understand the effect of hospital volume on outcomes and cost. This cutoff was chosen to account for the trend that 50% of RLs in the late phase were performed in hospitals with >25 annual volume. This cutoff is consistent with published data showing >20–25 annual lobectomies are necessary for improved outcomes at both the surgeon and hospital level (12-16).
To minimize selection bias and obtain more comparable patient cohorts for evaluation of the clinical and economic outcomes of RL compared with other surgical approaches, we performed a propensity score-matched (PSM) analysis for each period to minimize selection bias (17). Patient, surgeon, and hospital characteristics among surgical approaches were used to calculate the likelihood of receiving RL versus VL or OL via logistic regressions [variables used in PSM: age, gender (female, male), race (black, white, Hispanic, other), primary insurance type (Medicare, Medicaid, commercial, others), malignancy indication (primary neoplasm of lung, metastases other than lung), Charlson comorbidity score (0, 1–2, ≥3), obese status, pervious/current smoker (yes, no), year, attending surgeon specialty(thoracic surgeon, cardiovascular surgeon, others), attending surgeon volume in previous 12 month (≤10, 11–25, >25), bed size (0–199, 200–399, 400+), hospital location(Midwest, Northeast, South, West), hospital metropolitan location(urban, rural), hospital annual OL/VL/RL volume (≤25, >25), Listed in Table S2]. Based on the resulting propensity score, matched groups (1:1 match) were generated by greedy matching algorithm without replacement (18,19). The differences of characteristics between RL and OL or VL before and after matching were compared to assess the residual bias. Four separate propensity matches were performed (RL vs. VL in early and late periods, RL vs. OL in early and late periods).
Full table
Among the matched groups, chi-square tests/Fisher’s exact, and Wilcoxon rank-sum tests were used to examine the difference in categorical and right-skewed continuous outcomes. Statistical tests were two-sided, and P<0.05 was considered statistically significant. All analyses were conducted with SAS v9.4 (SAS Institute Inc., Cary, NC, USA).
Results
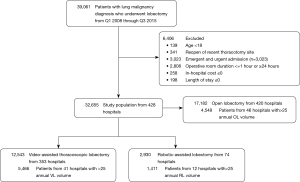
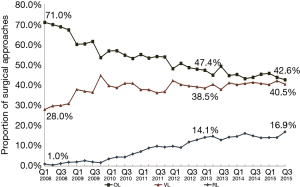
From 2008–2015 Q3, there were 39,061 lobectomies for lung neoplasm, and 6,406 patients were excluded. Of the remaining 32,655 lobectomies, there were 17,182 (52.6%) OL, 12, 543 (38.4%) VL, and 2,930 (9.0%) RL (Figure 1). The trend of surgical approaches is shown in Figure 2. There was a decline in OL (71.0% in Q1 2008 to 42.6% in Q3 2015, P<0.0001) and increase in RL (1.0% in Q1 2008 to 16.9% in Q3 2015, P<0.0001) and VL (28.0% in Q1 2008 to 40.5% in Q3 2015, P<0.0001).
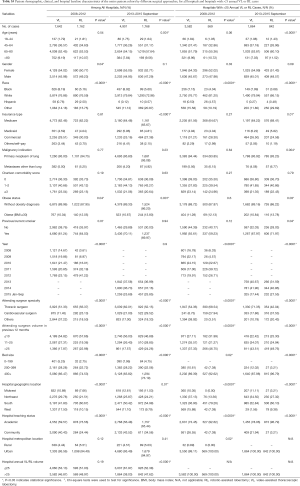
After propensity matching within each period, there were 1,136 cases each of VL and RL in the early period and 1,729 cases each in the late period. There were no significant differences between matched groups after PSM (before PSM, Table S3; after PSM, Table S4). Clinical outcomes and resource utilization of each modality are summarized in Table 1. In the early period, there was no difference in complications and in-hospital mortality between VL and RL groups, although VL was associated with a higher rate of open conversion than RL (10.8% VL vs. 6.4% RL, P=0.0003). Total OR time for RL was longer than VL by a median of 30 minutes (P<0.0001). Hospital LOS was a median of 5 days in each group, but RL showed a statistically significantly shorter LOS (P=0.01), attributable to the interquartile range (IQR) distribution. In the late period, there was no difference in intraoperative complications, but RL was associated with lower in-hospital and 30-day perioperative complications (38.9% RL vs. 44.3% VL, P=0.002). Conversion to open rates continued to be lower in the RL group (5.2% RL vs. 10.2% VL, P<0.0001), and OR time was longer in the RL group by a median of 18 minutes (P<0.0001). Hospital LOS remained statistically shorter with RL by 1 day (4 days RL vs. 5 days VL, P<0.0001). In-hospital mortality between RL and VL were similar.
Full table
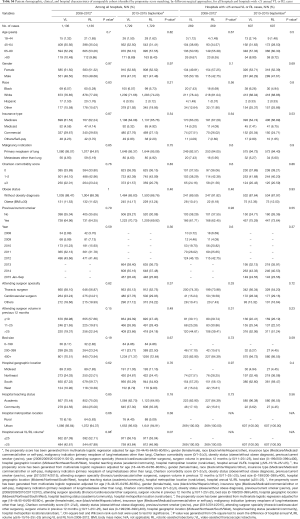
Full table
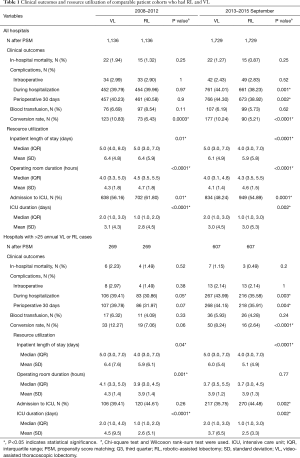
Full table
A subgroup analysis was performed limited to hospitals with >25 annual RL or >25 VL cases, and after PSM there were 269 matched cases in the early period and 607 matched cases in the late period (Table 1). There was no statistical difference in intraoperative complications in either period, but postoperative complications trended lower in the RL group in the early period (in-hospital P=0.05 and 30-day P=0.07). In the late period postoperative complications were statistically lower for RL (in-hospital P=0.003 and 30-day P=0.004). Conversion to open trended lower for the RL group in the early period (7.1% RL vs. 12.3% VL, P=0.06), and was significantly lower in the late period (2.6% RL vs. 8.2% VL, P<0.0001). Median LOS was significantly shorter for RL in both periods (4 days RL vs. 5 days VL for both periods; P=0.04 early period, P<0.0001 late period). OR time was shorter for RL in the early period (3.9 hours RL vs. 4.1 hours VL, P=0.001) and was similar in the late period (median: 3.7 hours RL vs. 3.7 hours VL, P=0.77). Mortality rates between groups were similar in both periods.
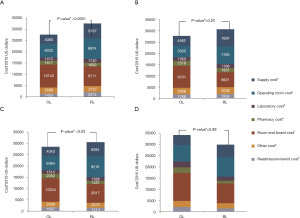
Analysis of costs between VL and RL cases for all hospitals and hospitals with >25 annual lobectomies are shown in Table 2. Costs of RL were higher than VL in both periods when hospital volume is not considered. Median 30-day perioperative direct cost of RL was $1,328 higher than VL in the early period (P<0.0001) and $1,279 higher vs. VL in the late period (P<0.0001). However, for hospitals with >25 annual cases, there was no difference in total costs for both the early and late periods (P=0.24 early period, P=0.18 late period). The median direct in-hospitalization cost of RL in higher-volume hospitals was lower than VL in the early period by $1,191 (P=0.03) and similar to VL in the late period (P=0.13). Figure 3 shows the breakdown of costs between RL and VL for both periods in all hospitals and those with >25 annual cases. When costs in all hospitals were analyzed, the higher total costs of RL were attributable to OR costs and supply costs. In hospitals with >25 annual RL or VL cases, RL was cost-neutral with VL due to the decreased room and board, supply and OR costs (early period P=0.53, late period P=0.59). Breakdown of room and board costs are shown in Figure S1.
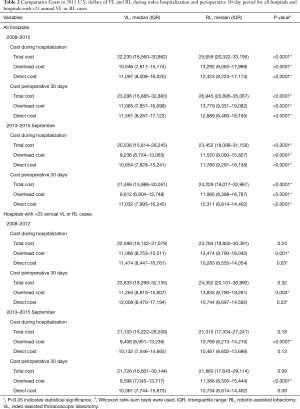
Full table
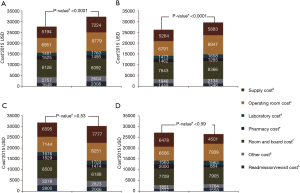

Similar analyses were performed on propensity-matched populations of OL and RL in both periods for all hospitals and those with >25 annual cases. Patient characteristics and outcomes are shown in Tables S5,S6. Longer OR times were noted in RL groups in all comparisons (all P<0.001). In the early period, the intraoperative complication rate was higher with RL (P=0.01) but was similar in hospitals with >25 annual cases. There was no difference in 30-day perioperative complications and in-hospital mortality regardless of volume. In the late period, RL was associated with a similar rate of intraoperative complications but a lower rate of in-hospital and 30-day perioperative complications. In both periods regardless of volume, RL was associated with a shorter LOS [median: 5 days RL vs. 7 days OL (early period), 4 days RL vs. 6 days OL (late period), both P<0.0001].
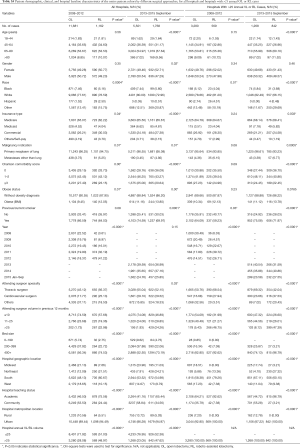
Full table
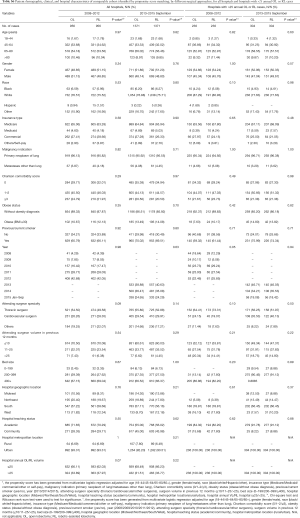
Full table
Cost analysis of OL and RL cases are shown in Tables S7,S8, and Figure S2. In the early period, the total cost of RL was significantly higher than OL for all hospitals and hospitals with >25 annual cases. In the late period, however, total costs of RL and OL were statistically similar for hospitals with >25 annual cases.
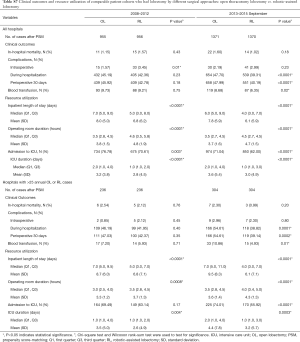
Full table
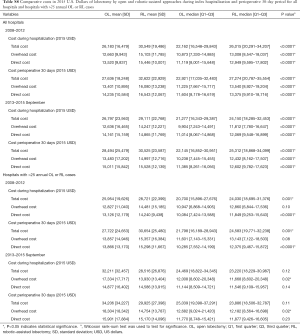
Full table
Discussion
During the past decade, there has been a major shift in the approach to lobectomy in the United States. The utilization of OL has declined by 40%, while minimally invasive lobectomy (RL+VL) has grown rapidly. This represents a significant paradigm shift in the surgical approach for lobectomy, as minimally invasive techniques now comprise the majority of cases. There have been two distinct trends during this period. Between 2008 and 2010 there was a major decline in OL as VL adoption increased. However, from 2011 the continued decrease of OL appears to have coincided with an increase in RL, while the rate of VL remained constant.
Comparative effectiveness analysis of RL and VL with a nationwide sample over the 8-year period identified improvements in outcomes associated with RL. In the early period from 2008–2012, clinical outcomes were similar between the groups, although there was a statistically shorter LOS with RL. These observations remained similar in higher volume hospitals during this early time period. While total cost was higher with RL when hospital volume is not considered, the cost difference was eliminated when hospitals had >25 RL cases annually. It is possible that the higher-volume hospitals performing RL had inherent efficiencies that reduced costs. Importantly, higher volume in RL was not necessary to achieve similar clinical outcomes during this early period when VL was a mature procedure compared with the early experience of RL before 2013.
During the late period from 2013–2015, RL was associated with improved clinical outcomes compared with VL, including decreased overall complications, less conversions, and shorter LOS. These observations were independent of hospital volume. This may reflect increased experience and standardization over time as surgeons collectively surmounted the learning curve of RL. The total cost of RL decreased from the early to late period, and similar to the early period, in hospitals with >25 annual cases, RL total cost was equivalent to VL, while still associated with improved clinical outcomes. In contrast to earlier publications (5), there is no difference in total costs between RL and VL when a modest annual volume is achieved. This cost neutralization occurred with improved clinical outcomes. This effect of higher lobectomy volume on lower cost was also demonstrated in a previous study (20).
RL was associated with longer OR duration compared with VL in both early and late adoption periods, although the median difference between the two approaches decreased from 30 to 18 minutes. This includes time from the patient wheeling in to wheeling out, which includes multiple factors other than pure operative time and may reflect increased set up time for robotic-assisted surgery. Notably, there was no time difference between VL and RL in hospitals with >25 annual cases in the late time period. This again suggests there is incremental improvement in efficiencies when a modest volume of cases is performed. This observation underscores the multifactorial contribution of institutional, OR, and surgical personnel efficiencies in the conduct of these operations.
The effect of hospital volume on outcomes is complex, and in many hospitals it is inseparable from the contribution of surgeon volume. In higher-volume hospitals, the surgeons’ volume in those hospitals was also higher, indicating that the two factors are intertwined. Published data indicate that hospitals with higher volume have better outcomes than their lower-volume counterparts (21,22). This is particularly true for minimally invasive lobectomy, as previous studies demonstrated that clinical outcomes of RL and VL improved with increases in hospital volume (23,24). In this study, we chose to focus on the hospital volume since operative outcomes are dependent on a team of personnel involved in the OR and in the postoperative setting, not just on the surgeon.
Similar observations on clinical outcomes were made for RL compared with OL, but currently there is little debate on the benefits of minimally invasive surgery, with minimally invasive surgery having several advantages over open lobectomy in perioperative outcomes (3). This study confirms that premise, with improved clinical outcomes with RL, especially in the more recent period. While total costs are higher for RL compared with OL overall, similar to the comparison with VL, when a modest volume of >25 annual cases is achieved, there was no difference in cost while preserving improved outcomes.
There are several limitations in a study using an administrative database. For example, ICU utilization likely reflects different surgeon and institutional practice patterns, admixed with unplanned ICU admissions. Also, there may be heterogeneity of cost structures between hospitals even after matching. The Premier Database does not have details about the tumor size or stage, and therefore selection bias could be present in cases selected for OL. However, similar size tumors and stage would be expected to be undertaken by both minimally invasive techniques. Finally, the study is observational, although propensity matching may mitigate some selection bias.
In summary, this study shows increased adoption and improved outcomes associated with RL compared with VL and OL over time, including lower overall complication rates and shorter LOS. Once a modest volume of >25 cases per year is achieved, these improvements persisted, along with a reduction in costs to render RL cost-neutral to VL and OL. These findings suggest RL is an effective and cost-comparable approach for pulmonary lobectomy for lung malignancies.
Acknowledgments
We gratefully acknowledge Ashfield Healthcare Communications (Middletown, CT, USA) for support in copyediting as well as Teresa Oblak, PhD, CMPP of Covance Market Access Services (Gaithersburg, MD) for implementing author-requested revisions and administrative tasks related to journal submission. This work, including access to the Premier database, statistical analysis, and copyediting support, was provided by Intuitive Surgical.
Funding: None.
Footnote
Conflicts of Interest: IS Sarkaria serves as the unpaid editorial board member of Journal of Thoracic Disease from Sep 2018 to Aug 2020 and he is consultant and teacher for Intuitive. IS Sarkaria: consultant and teacher for Intuitive. DS Oh is a part-time employee of Intuitive as a medical advisor and C Song and E Liu are full-time employees of Intuitive, during the conduct of the study. L Shi serves as a consultant to Intuitive. The other authors have no conflicts of interest to declare. All authors had full access to all of the data in the study and accept responsibility for the integrity of the data and the accuracy of the data analysis.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dziedzic D, Orlowski T. The role of VATS in lung cancer surgery: current status and prospects for development. Minim Invasive Surg 2015;2015:938430.
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16; discussion 2016-8.
- Dylewski MR, Ohaeto AC, Pereira JF. Pulmonary resection using a total endoscopic robotic video-assisted approach. Semin Thorac Cardiovasc Surg 2011;23:36-42. [Crossref] [PubMed]
- Park BJ, Flores RM. Cost comparison of robotic, video-assisted thoracic surgery and thoracotomy approaches to pulmonary lobectomy. Thorac Surg Clin 2008;18:297-300. vii. [Crossref] [PubMed]
- Yang CF, Sun Z, Speicher PJ, et al. Use and Outcomes of Minimally Invasive Lobectomy for Stage I Non-Small Cell Lung Cancer in the National Cancer Data Base. Ann Thorac Surg 2016;101:1037-42. [Crossref] [PubMed]
- Louie BE, Wilson JL, Kim S, et al. Comparison of video-assisted thoracoscopic surgery and robotic approaches for clinical stage I and stage II non-small cell lung cancer using the Society of Thoracic Surgeons Database. Ann Thorac Surg 2016;102:917-24. [Crossref] [PubMed]
- Yang HX, Woo KM, Sima CS, et al. Long-term Survival Based on the Surgical Approach to Lobectomy For Clinical Stage I Nonsmall Cell Lung Cancer: Comparison of Robotic, Video-assisted Thoracic Surgery, and Thoracotomy Lobectomy. Ann Surg 2017;265:431-7. [Crossref] [PubMed]
- Oh DS, Reddy RM, Gorrepati ML, et al. Robotic-Assisted, Video-Assisted Thoracoscopic and Open Lobectomy: Propensity-Matched Analysis of Recent Premier Data. Ann Thorac Surg 2017;104:1733-40. [Crossref] [PubMed]
- Kneuertz PJ, Singer E, D’Souza DM, et al. Hospital cost and clinical effectiveness of robotic-assisted versus video-assisted thoracoscopic and open lobectomy: A propensity score-weighted comparison. J Thorac Cardiovasc Surg 2019;157:2018-2026.e2. [Crossref] [PubMed]
- Kent M, Wang T, Whyte R, et al. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg 2014;97:236-42; discussion 242-4. [Crossref] [PubMed]
- Veronesi G, Novellis P, Difrancesco O, et al. Robotic assisted lobectomy for locally advanced lung cancer. J Vis Surg 2017;3:78. [Crossref] [PubMed]
- Melfi FM, Mussi A. Robotically assisted lobectomy: learning curve and complications. Thorac Surg Clin 2008;18:289-295. vi-vii. [Crossref] [PubMed]
- Reddy RM, Gorrepati ML, Oh DS, et al. Robotic-assisted versus thoracoscopic lobectomy outcomes from high-volume thoracic surgeons. Ann Thorac Surg 2018;106:902-8. [Crossref] [PubMed]
- McKenna RJ Jr. Complications and learning curves for video-assisted thoracic surgery lobectomy. Thorac Surg Clin 2008;18:275-80. [Crossref] [PubMed]
- von Meyenfeldt EM, Gooiker GA, van Gijn W, et al. The relationship between volume or surgeon specialty and outcome in the surgical treatment of lung cancer: a systematic review and meta-analysis. J Thorac Oncol 2012;7:1170-8. [Crossref] [PubMed]
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399-424. [Crossref] [PubMed]
- Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med 2014;33:1057-69. [Crossref] [PubMed]
- Parsons LS. Reducing bias in a propensity score matched-pair sample using greedy matching techniques. SAS Institute Inc. Available online: http://www2.sas.com/proceedings/sugi26/p214-26.pdf. Accessed July1, 2018.
- Harrison S, Tangel V, Wu X, et al. Are minimum volume standards appropriate for lung and esophageal surgery? J Thorac Cardiovasc Surg 2018;155:2683-2694.e1. [Crossref] [PubMed]
- Gruen RL, Pitt V, Green S, et al. The effect of provider case volume on cancer mortality: systematic review and meta-analysis. CA Cancer J Clin 2009;59:192-211. [Crossref] [PubMed]
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346:1128-37. [Crossref] [PubMed]
- Tchouta LN, Park HS, Boffa DJ, et al. Hospital volume and outcomes of robot-assisted lobectomies. Chest 2017;151:329-39. [Crossref] [PubMed]
- Park HS, Detterbeck FC, Boffa DJ, et al. Impact of hospital volume of thoracoscopic lobectomy on primary lung cancer outcomes. Ann Thorac Surg 2012;93:372-9. [Crossref] [PubMed]