
Lobectomy versus sublobar resection in patients with non-small cell lung cancer: a systematic review
Introduction
In recent years, thoracic surgery showed a significant boost technological evolution in the treatment of non-small cell lung cancer (NSCLC) associated with the development of genetic research for a targeted medical therapy (1-3). Currently, the wide resection is mandatory in order to obtain an oncologically adequate lung cancer (NSCLC) treatment; in fact, limited resection can be performed only if the general condition of patient is not particularly compliant (4,5). However, general well-being and lifestyle habits increased the average overall survival (6) to such that risk factors and comorbidities have been studied in over 100 years older age human (7). Older people benefit most from less invasive surgical approach also with the saving of lung parenchyma. However, regardless of the age and general status of the patients it is questionable whether major resection is still justified in early-stage NSCLC (8-10). Moreover, it is imperative to define risks and benefits of sublobar resections compared to the classic lobectomies not only in relation to the perspective survival (11,12). The purpose of the study was to evaluate the data in the literature regarding lobectomies and limited resections, in order to establish the correct indications for one or the other technique.
Methods
A search strategy using a combination of free-text words, relevant MeSH terms and appropriate filters was designed; the searching strategy was developed in MEDLINE (via PubMed) from 2014 until 2019. Records identified through our search strategy were imported into reference management software. The eligibility criteria were: “non-small cell lung cancer AND lobectomy AND/OR sublobar resection (anatomical segmentectomy, wedge resection) AND outcome”. Two authors worked independently to assess each identified study based on the eligibility criteria; when multiple studies contained overlapping data, a most informative study was included. Two independent reviewers and disagreements assessed the risk of bias were settled by discussion and consensus. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement was used to improve the report of this systematic review. The literature search was conducted by two reviewers independently. Any discrepancies in the final list of articles to be included were discussed and resolved by consensus. The following items were extracted from each study if available: first author’s surname, publication year, surgical strategy, recurrence, complications, overall survival.
Results
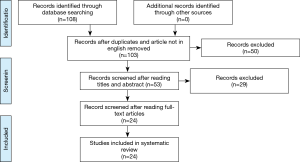
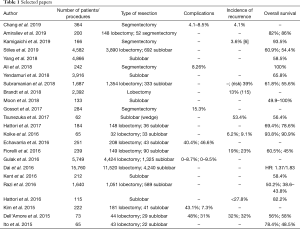
The selection of the articles was carried out by interrogating five databases: Medline, Scopus, CINAHL, Web of Science, Cochrane. The following search string was used: “non-small cell lung cancer AND lobectomy AND/OR sublobar resection (anatomical segmentectomy, wedge resection) AND outcome”. One-hundred eight results were obtained and, after removing of duplicates and article not in English, 103 articles were identified. Of these, 53 were found interesting after reading the title and the abstract. Afterwards, only 24 were evaluated relevant after reading the full text. This step was performed independently by two researchers. In case of doubt, a third independent researcher intervened from the previous ones. For the data analysis, 24 articles (13-36) were identified and taken into consideration (Figure 1). These provided overall information on 43,469 patients treated for NSCLC. Of these, 25,584 patients were treated with lobectomy and 17,885 were treated with sublobar resection (wedge or anatomical segmentectomy). Patients were divided into two distinct groups based on the surgical strategy and we have considered three parameters: complications, recurrence and overall survival. Sublobar resections compared to lobectomies showed (Table 1) an overall range in regard to: (I) the complication rate, between 0% and 46.6% and 0% and 48% respectively; (II) the incidence of recurrence, from 3.6% to 53.4% and 6.2% and 32% respectively; (III) the overall survival, from 38.6% to 100% and from 50.2% to 93.8% respectively. Therefore, from the analysis of individual studies was highlighted that sublobar resections are characterized by a reduced overall complication index but by a higher risk of developing local recurrence which it is translated into a lower overall survival index compared to lobectomies.
Full table
Discussion
Surgery is the best strategy treatment in NSCLC patients’ early stages (37), although the type of intervention is still debated. Pulmonary lobectomy represents the safest and oncologically correct choice (38,39). However, sublobar lung resections seem to reach the same levels of efficacy and accuracy compared to lobectomy (40) favoring the recruitment of elderly patients with poor functional respiratory reserve and/or high comorbidity index according to the saving of lung parenchyma (41). Indisputably, lobectomy seem to reduce the risk of local recurrence, improving overall survival (42,43). In our review the total number of patients enrolled in the various studies was 43,469 and data appear particularly significant. The first parameter taken into consideration is the complication rate. As can be deduced from Table 1, lobectomy shows a maximum value of 48% while sublobar resections show a maximum value of 46.6%. Echavarria et al. (27) displayed a higher complication rate compared to all Authors. This data can be explained by the characteristics of the study in which only patients with pneumological problems underwent segmentectomy and the complications were basically linked to basic respiratory insufficiency. Dell’Amore et al. (35) studied octogenarian patients with a high comorbidity index, responsible of 31% complications rate in sublobar resections. In fact, other comparative studies highlighted the complication rate not exceeding 15.3%. The higher rate of complications in lung lobectomies compared to sublobar resections can be explained with the greater stress on the cardiovascular system, due to the hemodynamic effects following the functional and anatomical reduction of the intrapulmonary vascular bed (44,45). Therefore, the careful patients selection associated with an accurate preoperative evaluation is essential to reduce the risks of intraoperative and postoperative complications. The second parameter evaluated was the incidence of recurrence. Percentages derived from the comparative articles in our study appear similar. However, considering also the studies analyzing the two techniques individually, it is clear that patients underwent sublobar resections have a greater possibility of recurrence than patients underwent lobectomy with maximum risk percentage equal to 53.4% and 32% respectively. This is due to the better oncological radicality obtainable with pulmonary lobectomy. In fact, during sublobar resections in not easy to sample the interlobar lymph nodes (station 11) whose cancer invasion can explain recurrences (46,47). Currently, has found consensus among the Authors the hypothesis about bronchial airspace involvement by locally spread of malignant cell elements (48-50). Kadota et al. (51) analyzed 411 adenocarcinoma patients (stage I) underwent lung resection. One hundred fifty-five of these (38%) showed spread tumor air space (STAS). The risk of developing recurrence in STAS patients underwent sublobar resections and lobectomy was 42.6% and 12.7% respectively. The third parameter considered in our study was the overall survival. One study (18) showed a survival equal to 100%. The bias is due to only 30-days survival is assessed in 242 patients underwent VATS sublobar resections. Moon et al. (22) studied the margin tumor ratio in 133 patients underwent sublobar resection and experienced 5-year recurrence-free survival (RFS) rate equal to 100% only in lepidic tumors with T <2 cm-N0M0. In non-lepidic tumor Groups RFS decreased up to 49.9%. Razi et al. (32) highlighted 5-year survival in NSCLC patients of 38.6% for wedge resection and of 43.8% for anatomical segmentectomy. Ito et al. (36) studied 65 NSCLC octogenarian patients, 43 of these treated by lobectomy while 22 by sublobar resection. Survival was higher in patients underwent lobectomy compared to limited resection with a rate equal to 78.4% and to 48.5% respectively. In conclusion, sublobar resections seem to be indicated in elderly patients with a high comorbidity index and reduced respiratory functional reserve. Pulmonary lobectomy still remains the safest and oncologically suitable method in patients with good performance status, reducing the risk of recurrence.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Mario Nosotti, Ilaria Righi and Lorenzo Rosso) for the series “Early Stage Lung Cancer: Sublobar Resections are a Choice?” published in Journal of Thoracic Disease. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.02.54). The series “Early Stage Lung Cancer: Sublobar Resections are a Choice?” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang L, Ma Q, Yao R, et al. Current status and development of anti-PD-1/PD-L1 immunotherapy for lung cancer. Int Immunopharmacol 2020;79:106088. [Crossref] [PubMed]
- Takada K, Toyokawa G, Shoji F, et al. The Significance of the PD-L1 Expression in Non-Small-Cell Lung Cancer: Trenchant Double Swords as Predictive and Prognostic Markers. Clin Lung Cancer 2018;19:120-9. [Crossref] [PubMed]
- Xu Y, Wan B, Chen X, et al. The association of PD-L1 expression with the efficacy of anti-PD-1/PD-L1 immunotherapy and survival of non-small cell lung cancer patients: a meta-analysis of randomized controlled trials. Transl Lung Cancer Res 2019;8:413-28. [Crossref] [PubMed]
- Bédat B, Abdelnour-Berchtold E, Perneger T, et al. Comparison of postoperative complications between segmentectomy and lobectomy by video-assisted thoracic surgery: a multicenter study. J Cardiothorac Surg 2019;14:189. [Crossref] [PubMed]
- Tsutani Y, Kagimoto A, Handa Y, et al. Wedge resection versus segmentectomy in patients with stage I non-small-cell lung cancer unfit for lobectomy. Jpn J Clin Oncol 2019;49:1134-42. [Crossref] [PubMed]
- Norman K, Klaus S. Veganism, aging and longevity: new insight into old concepts. Curr Opin Clin Nutr Metab Care 2020;23:145-50. [PubMed]
- Vaupel JW. Biodemography of human aging. Nature 2010;464:536-42. [Crossref] [PubMed]
- Divisi D, Imbriglio G, De Vico A, et al. Lung nodule management: a new classification proposal. Minerva Chir 2011;66:223-34. [PubMed]
- Lopes Pegna A, Picozzi G, Falaschi F, et al. Four-year results of low-dose CT screening and nodule management in the ITALUNG trial. J Thorac Oncol 2013;8:866-75. [Crossref] [PubMed]
- Munden RF, Chiles C, Boiselle PM, et al. Micronodules Detected on Computed Tomography During the National Lung Screening Trial: Prevalence and Relation to Positive Studies and Lung Cancer. J Thorac Oncol 2019;14:1538-46. [Crossref] [PubMed]
- Landreneau RJ, Schuchert MJ. Is segmentectomy the future? J Thorac Dis 2019;11:308-18. [Crossref] [PubMed]
- Landreneau RJ, Sugarbaker DJ, Mack MJ, et al. Wedge resection versus lobectomy for stage I (T1 N0 M0) non-small-cell lung cancer. J Thorac Cardiovasc Surg 1997;113:691-8. [Crossref] [PubMed]
- Chang CC, Yen YT, Lin CY, et al. Single-port video-assisted thoracoscopic surgery subsegmentectomy: The learning curve and initial outcome. Asian J Surg 2020;43:625-32. [PubMed]
- Amiraliev AM, Pikin OV, Ryabov AB, et al. Segmentectomy in patients with primary pulmonary malignancies. Khirurgiia 2019.5-12. [PubMed]
- Kamigaichi A, Tsutani Y, Fujiwara M, et al. Postoperative Recurrence and Survival After Segmentectomy for Clinical Stage 0 or IA Lung Cancer. Clin Lung Cancer 2019;20:397-403.e1. [Crossref] [PubMed]
- Stiles BM, Mao J, Harrison S, et al. Sublobar resection for node-negative lung cancer 2-5 cm in size. Eur J Cardiothorac Surg 2019;56:858-66. [Crossref] [PubMed]
- Yang H, Li X, Shi J, et al. A nomogram to predict prognosis in patients undergoing sublobar resection for stage IA non-small-cell lung cancer. Cancer Manag Res 2018;10:6611-26. [Crossref] [PubMed]
- Ali J, Haiyang F, Aresu G, et al. Uniportal Subxiphoid Video-Assisted Thoracoscopic Anatomical Segmentectomy: Technique and Results. Ann Thorac Surg 2018;106:1519-24. [Crossref] [PubMed]
- Yendamuri S, Dhillon SS, Groman A, et al. Effect of the number of lymph nodes examined on the survival of patients with stage I non-small cell lung cancer who undergo sublobar resection. J Thorac Cardiovasc Surg 2018;156:394-402. [Crossref] [PubMed]
- Subramanian M, McMurry T, Meyers BF, et al. Long-Term Results for Clinical Stage IA Lung Cancer: Comparing Lobectomy and Sublobar Resection. Ann Thorac Surg 2018;106:375-81. [Crossref] [PubMed]
- Brandt WS, Bouabdallah I, Tan KS, et al. Factors associated with distant recurrence following R0 lobectomy for pN0 lung adenocarcinoma. J Thorac Cardiovasc Surg 2018;155:1212-24.e3. [Crossref] [PubMed]
- Moon Y, Lee KY, Park JK. Margin Width of Resected Lepidic Lung Cancer Does Not Affect Recurrence After Sublobar Resection. World J Surg 2018;42:1449-57. [Crossref] [PubMed]
- Gossot D, Lutz JA, Grigoroiu M, et al. Unplanned Procedures During Thoracoscopic Segmentectomies. Ann Thorac Surg 2017;104:1710-7. [Crossref] [PubMed]
- Tsunezuka H, Kato D, Okada S, et al. Surgical outcome of wide wedge resection in poor-risk patients with clinical-N0 non-small cell lung cancer. Gen Thorac Cardiovasc Surg 2017;65:581-6. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Takamochi K, et al. Surgical resection for clinical-Stage I radiological pure-solid lung cancer that met the current high risk criteria. Jpn J Clin Oncol 2017;47:630-8. [Crossref] [PubMed]
- Koike T, Koike T, Sato S, et al. Lobectomy and limited resection in small-sized peripheral non-small cell lung cancer; Niigata Chest Surgery Research Group. J Thorac Dis 2016;8:3265-74. [Crossref] [PubMed]
- Echavarria MF, Cheng AM, Velez-Cubian FO, et al. Comparison of pulmonary function tests and perioperative outcomes after robotic-assisted pulmonary lobectomy vs segmentectomy. Am J Surg 2016;212:1175-82. [Crossref] [PubMed]
- Fiorelli A, Caronia FP, Daddi N, et al. Sublobar resection versus lobectomy for stage I non-small cell lung cancer: an appropriate choice in elderly patients? Surg Today 2016;46:1370-82. [Crossref] [PubMed]
- Gulack BC, Yang CJ, Speicher PJ, et al. A Risk Score to Assist Selecting Lobectomy Versus Sublobar Resection for Early Stage Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;102:1814-20. [Crossref] [PubMed]
- Dai C, Shen J, Ren Y, et al. Choice of Surgical Procedure for Patients With Non-Small-Cell Lung Cancer ≤ 1 cm or > 1 to 2 cm Among Lobectomy, Segmentectomy, and Wedge Resection: A Population-Based Study. J Clin Oncol 2016;34:3175-82. [Crossref] [PubMed]
- Kent MS, Mandrekar SJ, Landreneau R, et al. A Nomogram to Predict Recurrence and Survival of High-Risk Patients Undergoing Sublobar Resection for Lung Cancer: An Analysis of a Multicenter Prospective Study (ACOSOG Z4032). Ann Thorac Surg 2016;102:239-46. [Crossref] [PubMed]
- Razi SS, John MM, Sainathan S, et al. Sublobar resection is equivalent to lobectomy for T1a non-small cell lung cancer in the elderly: a Surveillance, Epidemiology, and End Results database analysis. J Surg Res 2016;200:683-9. [Crossref] [PubMed]
- Hattori A, Takamochi K, Matsunaga T, et al. Oncological outcomes of sublobar resection for clinical-stage IA high-risk non-small cell lung cancer patients with a radiologically solid appearance on computed tomography. Gen Thorac Cardiovasc Surg 2016;64:18-24. [Crossref] [PubMed]
- Kim D, Ferraris VA, Davenport D, et al. Outcomes of lobar and sublobar resections for non-small-cell lung cancer: a single-center experience. South Med J 2015;108:230-4. [Crossref] [PubMed]
- Dell'Amore A, Monteverde M, Martucci N, et al. Lobar and sub-lobar lung resection in octogenarians with early stage non-small cell lung cancer: factors affecting surgical outcomes and long-term results. Gen Thorac Cardiovasc Surg 2015;63:222-30. [Crossref] [PubMed]
- Ito H, Nakayama H, Yamada K, et al. Outcomes of lobectomy in 'active' octogenarians with clinical stage I non-small-cell lung cancer. Ann Thorac Cardiovasc Surg 2015;21:24-30. [Crossref] [PubMed]
- Gaudet MA, D'Amico TA. Thoracoscopic Lobectomy for Non small Cell Lung Cancer. Surg Oncol Clin N Am 2016;25:503-13. [Crossref] [PubMed]
- Speicher PJ, Gu L, Gulack BC, et al. Sublobar Resection for Clinical Stage IA Non-small-cell Lung Cancer in the United States. Clin Lung Cancer 2016;17:47-55. [Crossref] [PubMed]
- Cohen C, Al Orainy S, Pop D, et al. Anatomical pulmonary resections for primary lung cancer in octogenarians within a dedicated care protocol. J Thorac Dis 2019;11:3732-7. [Crossref] [PubMed]
- Liu T, Liu H, Li Y. Early lung cancer in the elderly: sublobar resection provides equivalent long-term survival in comparison with lobectomy. Contemp Oncol (Pozn) 2014;18:111-5. [Crossref] [PubMed]
- Zhang Z, Feng H, Zhao H, et al. Sublobar resection is associated with better perioperative outcomes in elderly patients with clinical stage I non-small cell lung cancer: a multicenter retrospective cohort study. J Thorac Dis 2019;11:1838-48. [Crossref] [PubMed]
- Eguchi T, Kameda K, Lu S, et al. Lobectomy Is Associated with Better Outcomes than Sublobar Resection in Spread through Air Spaces (STAS)-Positive T1 Lung Adenocarcinoma: A Propensity Score-Matched Analysis. J Thorac Oncol 2019;14:87-98. [Crossref] [PubMed]
- Weiss K, Rochfort MM. Spread through air spaces-positive T1 lung adenocarcinoma: is lobectomy associated with better outcomes than sublobar resection? Ann Transl Med 2019;7:S126. [Crossref] [PubMed]
- Mageed NA, El-Ghonaimy YA, Elgamal MA, et al. Acute effects of lobectomy on right ventricular ejection fraction and mixed venous oxygen saturation. Ann Saudi Med 2005;25:481-5. [Crossref] [PubMed]
- Seok Y, Cho S, Lee JY, et al. The effect of postoperative change in bronchial angle on postoperative pulmonary function after upper lobectomy in lung cancer patients. Interact Cardiovasc Thorac Surg 2014;18:183-8. [Crossref] [PubMed]
- Song KJ, Flores RM. Is survival after sublobar resection vs. lobectomy made equivalent by extent of lymphadenectomy? Ann Transl Med 2019;7:S191. [Crossref] [PubMed]
- Stiles BM, Mao J, Harrison S, et al. Extent of lymphadenectomy is associated with oncological efficacy of sublobar resection for lung cancer ≤2 cm. J Thorac Cardiovasc Surg 2019;157:2454-65.e1. [Crossref] [PubMed]
- Shiono S, Endo M, Suzuki K, et al. Spread Through Air Spaces Is a Prognostic Factor in Sublobar Resection of Non-Small Cell Lung Cancer. Ann Thorac Surg 2018;106:354-60. [Crossref] [PubMed]
- Toyokawa G, Yamada Y, Tagawa T, et al. Significance of Spread Through Air Spaces in Resected Lung Adenocarcinomas With Lymph Node Metastasis. Clin Lung Cancer 2018;19:395-400.e1. [Crossref] [PubMed]
- Liu H, Yin Q, Yang G, et al. Prognostic Impact of Tumor Spread Through Air Spaces in Non-small Cell Lung Cancers: a Meta-Analysis Including 3564 Patients. Pathol Oncol Res 2019;25:1303-10. [Crossref] [PubMed]
- Kadota K, Nitadori J, Sima CS, et al. Tumor Spread through Air Spaces is an Important Pattern of Invasion and Impacts the Frequency and Location of Recurrences after Limited Resection for Small Stage I Lung Adenocarcinomas. J Thorac Oncol 2015;10:806-14. [Crossref] [PubMed]


