Uniportal VATS approach to sub-lobar anatomic resections: literature review and personal experience
Pulmonary lobectomy has been considered as the standard surgical procedure for lung cancer, since the prospective randomized controlled study published by the U.S. Lung Cancer Study Group in 1995 (1) showed a lower local recurrence rate and a better long-term survival rate when performing lobectomies compared to sublobectomies.
Nevertheless, technical advances in non small cell lung cancer (NSCLC) early diagnosis and in thoracic surgery techniques have changed the treatment approach to early stage NSCLC (2,3). The extensive application of high-resolution computed tomography (HRCT) in NSCLC screening programmes for high-risk former smokers has increased the detection of an even greater number of tiny NSCLC (<2 cm) and pure ground-glass opacity (GGO) lesions (4). This phenomenon has led to a modification of the 8th Edition of TNM classification of lung cancer (5), introducing tumors of ≤1 cm that are classified into a new T1a group, and had also led to the inclusion of the presence of GGO lesions as a radiological classification criterium suggestive of minimally invasive or noninvasive NSCLC (6).
This scenario has renewed surgeons’ interest in sublobar resections, not only for high-risk but also for fitting patients with early stage I NSCLC.
Simultaneously, anatomical segmentectomies (introduced as a surgical approach to benign lesions in 1939) has reemerged as a treatment option for such less-invasive NSCLC.
Compared with wedge resections, typical segmentectomies can ensure an adequate distance of the lesion from the margins, thereby reducing tumor recurrence and improving long-term survival (7) resulting in the most tailored surgical approach in the landscape of personalized medicine.
Although more evidence is claimed to definitively establish the role of intentional segmentectomies in the surgical treatment of early stage NSCLCs, a recent meta-analysis disclosed that segmentectomies produce similar survival outcomes compared to lobectomies in patients affected by stage I NSCLC (8).
Additionally, patients with multifocal partially solid tumors/purely GGO lesions may also be perfect candidates for segmentectomies, as they may require multiple and/or repeated resections (9) as claimed by Gonzalez-Rivas (10), who described “en bloc segmentectomies (2 or 3 combined segments)” for several GGOs located in different adjacent segments or located in the intersegmental plane.
In case of intentional segmentectomies, patient selection is of fundamental importance. The usual indications for a lung segmentectomy include: small (<1 cm) GGO lesions (<50%); tumor less than 2 cm in diameter without thoracic lymph node involvement and non malignant lung disease, where it’s necessary to preserve normal lung parenchyma.
In properly selected patients, even more evidences suggest that anatomic segmentectomies represent a valid alternative to lobectomies, offering a better quality of postoperative life, a lower operative morbidity and increased possibility for the patient to undergo a second or third surgical resection in case of a newly diagnosed NSCLC, without compromising oncological principles (11-23).
Surgeons who properly select tumors and achieve adequate margins and lymph node sampling describe comparable outcomes between segmentectomies (24,25) and lobectomies.
The role of extensive lymph node dissection in terms of recurrence-free survival and postoperative treatment planning remains of supreme importance also for segmentectomies. Results from National database studies demonstrate that a worse outcome in segmentectomies is often associated with a less extensive mediastinal lymph node dissection (26,27).
Since the traditional multiport video-assisted thoracoscopic surgery (MP-VATS) has become the standard approach for thoracic procedures (28), many reports address the role of thoracoscopic segmentectomy as the best lung-sparing operation for early stage NSCLC.
Compared with lobectomy, VATS segmentectomy achieves equivalent oncological results with less postoperative pain, lower post-operative morbidity and mortality (29) associated with a shorter chest tube duration (30) and hospitalization (31) and lower medical costs (32,33).
Since the earliest report of uniportal VATS (U-VATS) segmentectomy published by Diego Gonzalez-Rivas in 2012 (34), the feasibility and the advantages of U-VATS have been described in different fields of thoracic surgery. Despite the initial mistrust towards this technique, U-VATS has progressively emerged as a less invasive technique than the conventional MP-VATS, and various surgical groups have adopted this technique worldwide (35).
U-VATS has shown to be safe and feasible, even in complex procedures such as sleeve resections (36,37), segmentectomies (34,38,39) and vascular reconstructions (40,41).
Potential advantages for patients undertaking surgery with U-VATS technique are: reduced intercostal nerve injury compared with traditional surgery and less postoperative pain. During U-VATS procedures, the insertion of the camera and surgical devices is perpendicular to the plane of the chest, causing less compression on the intercostal nerves and significantly reducing subsequent paresthesia. In contrast, MP-VATS, with more incisions at different intercostals spaces, damages intercostal nerves at several sites worsening postoperative pain. Jutley et al. (42) found a significant difference in visual analogue scale (VAS) pain scores in patients who underwent U-VATS procedures compared with those who were treated with MP-VATS technique for spontaneous pneumothorax. The uniportal group had a lower median score of 0.4 (visual analogue range 0–4) while the three-port technique group reported a median pain score of 0.8 (P=0.06, Mann-Whitney test). The maximum score trend was similar (1.4 vs. 2.6, respectively; P<0.001, Mann-Whitney test). A higher residual pain score (VAS 0.5) was found in the MP-VATS group when compared to U-VATS (VAS 0.3). Regarding neurological complications, 86% of uniportal patients reported no symptoms and the other 14% only referred mild ‘numbness’ or ‘swelling’. On the contrary, only 42% of patients in the three-port group reported no symptoms, and a similar number experienced ‘numbness’. Although no randomized studies have been published proving that U-VATS is better than the conventional approach or even thoracotomy, meta-analysis studies indicate that conventional VATS has better results than thoracotomy, but U-VATS reveales more favorable outcomes than the multiportal approach (43). There is a statistically significant reduction in the length of hospital stay (6.2±2.6 vs. 6.7±3.4 days, P<0.0001), a reduction of postoperative drainage stay (4.5±2.2 vs. 5.4±2.9 days, P=0.0006) and overall morbidities (12.0% vs. 13.7%, P=0.009) for patients undergoing U-VATS lobectomies compared to the multiportal approach.
Segmentectomy techniques comparison
The better postoperative outcomes of U-VATS versus open thoracotomy segmentectomy has been well illustrated by Surendrakumar (44), who retrospectively analyzed 86 consecutive patients who underwent open or U-VATS segmentectomy. The author found similar results in terms of surgical outcomes, equivalent R1 resection margins and nodal stations exploration. No postoperative deaths were described in the U-VATS group while one was reported in the open-surgery group. Patients in the U-VATS group had a shorter hospital stay [median of 4 days (range, 1–15 days) vs. median of 6 days (range, 3–27 days), P=0.01], without any difference in the incidence of complications or readmissions to the hospital over time. Many studies have been conducted with the aim to assess the advantages of U-VATS vs. MP-VATS. Ji (45) evaluated 458 patients who received U-VATS or MP-VATS major anatomical resections: there were no differences between the two groups in the number (P=0.278) and stations (P=0.564) of lymph nodes sampled, postoperative morbidity (P=0.414) or mortality (P=0.246), and in pain scores on the third day after surgery (P=0.630). Surgical time was longer in the U-VATS group (P=0.042) with a greater intraoperative blood loss (P<0.001), but the conversion rate was even higher in the MP group (P=0.018). Patients in the U-VATS group experienced a shorter chest tube stay (P=0.012), a shorter postoperative hospitalization (P=0.005) and lower pain scores on the first (P=0.014) and second (P=0.006) day after surgery. Authors concluded that U-VATS lobectomies and anatomical segmentectomies are technologically more demanding than when approached through MP- VATS, but the least invasive surgery leads to experiencing less pain in the early postoperative period. Similar results were described by Han et al. (46) on a total of 45 patients who underwent pulmonary segmentectomy by U-VATS or MP-VATS between March 2006 and October 2015. The surgical time in the U-VATS segmentectomy group (148±65 min) was longer than in the MP-VATS segmentectomy group (107±68 min), although this difference was not statistically significant (P=0.073). The number of resected lymph nodes (n=24) was higher (P=0.031) in a relatively small population (n=3) in the MP-VATS group compared to the U-VATS group. Although no significant difference was registered in intraoperative events (P=0.412) and prolonged air leak (>5 days) (P=0.610), postoperative morbidity (P<0.001) and hospital stay (P=0.029) were lower in the U-VATS group. Lee (47) retrospectively analyzed 84 patients who underwent a U-VATS or MP-VATS anatomic segmentectomy: despite anesthesia and surgical times were similar in the two groups (215 vs. 220 minutes, respectively, P=0.276 and 180 vs. 198 minutes, respectively, P=0.396), blood loss (50 vs. 100 mL, P=0.013), chest tube duration (2 vs. 3 days, P=0.003) and hospital stay [4 days (range, 1–14 days) vs. 4 days (range, 1–62 days), P=0.011] were significantly lower in the uniportal group. The number of dissected lymph nodes tended to be lower in the uniportal group (5 vs. 8, P=0.056). Wang (48), in a propensity-matched analysis on 223 patients, compared the perioperative outcomes of U-VATS and MP-VATS lobectomies and segmentectomies; after propensity matching, 46 patients resulted eligible in each group. No significant differences were found between the two groups in terms of length of hospital stay and complication rate, but U-VATS lobectomies and segmentectomies were associated with shorter operative time (P=0.029), higher numbers of sampled lymph nodes (P=0.032), and less intraoperative blood loss (P=0.017). Moreover, the width of the wound resulted being a better outcome in favour of U-VATS vs. MP-VATS (39). The feasibility and safety of U-VATS has also been assessed during complicated segmentectomies. A complicated segmentectomy does not belong to the conventional segmentectomy and it’s is rarely discussed in surgical literature. The comparison between U-VATS and MP-VATS in complicated segmentectomies was reported by Chen (49), who evaluated 96 U-VATS and 68 MP-VATS complicated segmentectomies between July 2010 and March 2017. After propensity matching, the author analyzed 56 patients in each group. Despite surgical times and blood loss were not significantly different between the two groups, U-VATS complicated segmentectomies showed a shorter duration of pleural drainage and postoperative hospital stay (2.8 vs. 3.6 days and 4.2 vs. 5.3 days, respectively) (P<0.01). Both the intraoperative and postoperative complication rates were not significantly different as well. No 30-day mortality was observed in the series.
Learning curve
VATS segmentectomy requires excellent understanding of the hilar/mediastinal anatomy, much more than in lobectomies, to properly identify and expose the segmental structures, and more ability in handling instrumentation. Segmentectomies are not all equivalent; they can be classified into two groups: typical and atypical segmentectomies. Typical segmentectomies are simple procedures, e.g., right S1 and lingular segmentectomies (where parenchymal division involves 1 or 2 planes), while atypical ones are challenging procedures (e.g., S3 or S7, S8 segmentectomies) with the need of two or more intersegmental plane resections.
Typical segmentectomies have been relatively frequently described in a VATS (50) setting while, in contrast, atypical segmentectomies are technically feasible but remain challenging and reports are still limited (51,52).
Due to the complex anatomic orientation, mainly in case of atypical segmentectomies, it’s absolutely crucial to plan the operation by performing, preoperatively, a HRCT scan with 3D reconstruction to assess the lateral, the axial and the sagittal views in order to recognise the segmental anatomical structures (including veins, arteries and bronchus) and identify any anatomic variation (53).
Despite segmentectomies remain a challenging procedure, especially in minimally invasive surgery, data from surgical literature seem to demonstrate that the learning curve of U-VATS segmentectomies may be shorter than the MP-VATS one.
In fact, while McKenna (54) and Petersen (55) suggested a length of learning curve for MP-VATS lobectomy of at least 50 procedures, Hernandez-Arenas (56), evaluating U-VATS lobectomy and segmentectomy learning curve in a high volume training Center, found that one surgeon can complete the learning curve after only 30 cases. Cheng et al. (57), analyzing data from a retrospective observational study on 40 patients who underwent U-VATS segmentectomy with a cumulative summative analysis and one-way ANOVA, confirmed that the learning curve resulted completed after 33 patients, with no conversion to three-port VATS, two-port VATS or open thoracotomy. In our experience (58) the cumulative sum analysis on 46 consecutive patients who underwent U-VATS major lung resection showed that the learning curve resulted completed after 25 cases. Using the cut-off of 25 patients, the whole populations was divided in group A (first 25 patients of the experience) and group B (the last 18 patients). The mean operative time in group B was significantly shorter than in group A (164.00±24.46 vs. 191.40±50.45 min, respectively, P=0.04). There were no differences in demographic characteristics, number of removed lymph nodes, chest tube duration and hospital stay among the two groups. The number of conversions was higher in group A (4 vs. 0; P=0.07), as the number of major complications, like reinterventions for bleeding (2 vs. 0; P=0.22). There was no postoperative 30-day-related death.
No intraoperative vessel injury or anatomical variants causing significant changes in the surgical plan was reported by Duan et al. (59), who evaluated 156 consecutive patients with lung lesions who received anatomical pulmonary segmentectomy by U-VATS between 2015 and 2016. There was a significant correlation between the surgical time and the cumulative sum of the number of VATS segmentectomies performed by the surgeon (r=−0.593, P<0.001). The average surgical time of 123 min reported by the Author resulted absolutely in line with the similar surgical literature (38,60,61), while a progressive reduction in operative time directly correlated with the number of procedures performed by each operator.
Key points: localization of small/non-palpable lung lesions and intersegmental plane identification
VATS segmentectomy is particularly valuable for the resection of small, non-visible or non-palpable tumors. During mono o multiportal VATS procedures, though, it’s often difficult to palpate and identify small and deeply located nodules. Many techniques have been proposed to overcame this obstacle: intraoperative ultrasonography (62), hook-wire (63), hook-wire and lipiodol or a radioisotope (64), CT fluoroscopy (65), electromagnetic navigation bronchoscopy (66,67), combined techniques in hybrid operation theatres (68), preoperative injection of drugs, dyes (69-72) radionuclides (73), and contrast medium injection (lipiodol, barium) (74,75). Each of these localization methods has their own advantages and drawbacks. At present, the most commonly used technique is CT-guided hookwire localization (63), but the hookwire dislodgement before surgery is, unfortunately, relatively frequent. Thaete et al. (76) reported a dislodgement rate of 12% in cases without an initial pneumothorax and of 33% in cases with pneumothorax occurrence. According to Chen et al. (63), situations responsible for dislodgment of the hookwire may be: (I) during transfer to the operating room, the hookwire can be pulled as the chest wall and shoulder girdle move in relation to the lung; (II) the friction between the chest wall and the hookwire makes the hookwire dislodge during surgical deflation of the lung; (III) when the surgeons apply traction on the hookwire to tent the lung to facilitate resection, the hookwire can dislodge. In addition, dislodgement of the hookwire is related to the size and depth of the lesion. The most common complication of hookwire localizzation is pneumothorax, which can occur during the procedure itself. The incidence of pneumothorax in these cases is around 18% (63).
Intraoperative ultrasonography (62) with convex probe allows the surgeon to localize subcentimetric lesions, but the depth of the nodules significantly influences the quality of the images (excellent resolution up to 1.5 cm). The complete collapse of the lung or the use of high frequencies can help to better visualize the targets.
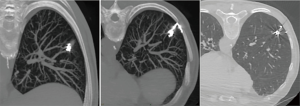
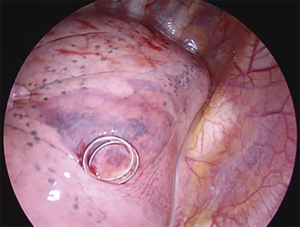
Many techniques based on preoperative injection of drugs, dyes (69-72), radionuclides (73), and contrast medium injection (lipiodol, barium) (74,75) have been proposed. The main disadvantage of these procedures is the necessity to perform surgery immediately after localization. The electromagnetic navigation bronchoscopy (66,67) is directly performed in the operating room, with the aim to identify small peripheral lesions by injecting methylene blue, which results visible on the visceral pleura thus allowing the affected segment to be resected. This technique doesn’t need facilities such as a fluoroscope, radiotracers, radioprobes, hookwires or coils and has the obvious advantage of not exposing the patient and the surgeons to radiations; on the other hand, methylene blue has the tendency to spread in the parenchyma adjacent to the injection site and this makes it of primary importance to perform surgery immediately after the localizzation. When the localization is performed with Lipiodol (74), the tracer can be aspirated at alveolar level, causing acute damage and inflammation. Additionally, in patients affected by silicosis, colour might be difficult to visualize. Localization with barium (75) under fluoroscopic guidance offers the advantage of allowing greater flexibility in the organization of the operating room; it remains in site longer than other dyes and, interestingly, the surgeon can be able to palpate the barium balls during VATS. However, parenchymal damage can occur, creating an acute inflammation with neutrophils and histiocytes infiltration in the barium containing site, which can alter histological diagnosis, especially in the case of pure GGOs. In the attempt to overcome all these obstacles, we recently described our experience with a nouvelle technique in preoperative computed tomography (CT)-guided embolization microcoil (14-mm diameter ×14 cm length, synthetic fiber-coated, stainless steel, Cook, Bloomington, IN, USA) localization of GGO nodules in 30 patients undergoing uniportal VATS lung resection (77). The coil has the peculiarity to be deployed beneath the nodule and partially along the transpulmonary route till the pleural space, with the distal tail of the coil left above the visceral pleura surface, serving as a guidance for the surgeon (Figure 1). No special instrumentation as fluoroscopy is needed in the operating theatre to identify the coil. The mean procedure time resulted being of 35±15 minutes. In 5 cases, the localization procedure was complicated by asymptomatic pneumothorax and in 1 case the pneumothorax required chest tube insertion. In all cases, conversion to thoracotomy was avoided as all nodules were identified and resected through uniportal VATS. The advantages of the use of microcoils, usually adopted for vessels embolization, are numerous: they are commonly used, easy to acquire and inexpensive compared with radionuclides; they can also be safely kept in the human body for days; after implantation, coils can be felt like a certain degree of hardness in the lung parenchyma and they are radiopaque, all aspects that enable finding the position by visual inspection, palpation, and, if required, fluoroscopy during surgery; coil placement is easy and has good repeatability (78) (Figure 2).
Recognition of the intersegmental plane is a key step in segmentectomy. Classically, the intersegmental veins are considered the anatomical landmarks during the dissection of the central portion of the intersegmental plane, but no anatomic landmarks are present on the visceral pleura, making the completion of segmentectomies sometimes very hard. Moreover, the identification of the intersegmental plane during segmentectomy is crucial to achieve the oncological proper excision with respect to adequate margins and the conservation of normal lung parenchyma. This step still remains the most challenging part of the procedure, especially in patients with chronic obstructive pulmonary disease (COPD), where the hyperinflated state of the lung parenchyma makes the intersegmental plane identification even more challenging. The historically most utilized technique for the identification of intersegmental planes during open and VATS procedures is the creation of a demarcation line between the target segment and residual parenchyma by inflating the lung after the segmental bronchus closure (79).
However, the presence of collateral canals that permit retrograde inflation of the target segment despite the closure of the segmental bronchus, may result in a difficult identification of the intersegmental line especially in COPD patients, where the emphysematous lungs often become overinflated. Several methods have been described in the effort to overcome these difficulties. In 2007, Okada et al. proposed a bronchoscopic selective ventilation of the segmental bronchus (60), while Kamiyoshihara et al. (80) proposed the inflation of the involved segment only by instilling oxygen through a butterfly needle into the bronchus subtending the segment. In order to obtain the demarcation between inflated and deflated lung, leaving only the target segment inflated, a slipknot bronchial ligation of the segmental bronchus during VATS segmentectomy was proposed by Oizumi et al. (81) in 2014. As an alternative to lung inflating procedures, many authors evaluated the efficacy of dyes injection into the segmental pulmonary artery (82) or peripheral segmental bronchus following the ligation of the pulmonary vein (83,84). Kato and colleagues (85) described the technique of 3-dimensional lung segmental color mapping from multislice CT angiography to understand the individual lung segmental anatomy and variations of the segmental vessels and bronchi. A 98% completion rate for segmentectomy using 3-dimensional reconstruction images was reported. Also, they emphasized that the intersegmental vein could be a guide for the parenchymal dissection (86).
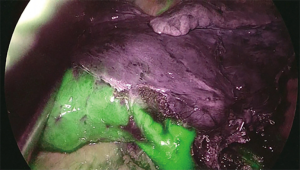
A novel technique is the virtual assisted lung mapping (VAL-MAP) (87) that allows for bronchoscopic multi-spot dye markings to provide “geometric information” to the lung surface, using three-dimensional virtual images. It allows the surgeon to constantly reconfirm the actual anatomy, thereby avoiding the misidentification of vessels and preventing inadequate resections. In addition, the VAL-MAP technique allows intraoperative guidance and seems to be valuable for technically challenging segmentectomies. In 2009, an experimental study conducted on pigs (88) showed the feasibility of intersegmental plane identification under near infrared (NIR) imaging and indocyanine green (ICG) administration. ICG is a NIR fluorescent dye. The technique is based on the evaluation of blood supply to the lung by using the arterial segmentation instead of the traditional bronchial segmentation. ICG is administered intravenously through a peripheral vein after segmental artery ligation and segmental boundaries are evaluated under NIR thoracoscopy. The lung appears on the monitor as divided into two areas, blue and white, according to the blood flow (blue is the vascularized lung, white is the devascularized parenchima). On the wave of this preliminary experience, Misaki and colleagues (89) reported their experience on patients who underwent NIR thoracoscopy after intravenous ICG injection. This method, depending on blood flow and avoiding reinflation of the lung, resulted suitable not only for patients with normal parenchyma, but also and especially for those with emphysematous lungs. On the basis of the encouraging results published on the use of NIR/ICG in intersegmental plane identification MP-VATS (90) and robotic segmentectomy (86), we decided to apply this technique to U-VATS segmentectomy (91). After ligation and transection of segmental vessels and bronchus, the camera is switched from standard white light to NIR light mode via a footswitch. Five to 7-mL bolus of ICG (2.5 mg/10 mL) depending on the weight of the patient and lung parenchyma status is intravenously administered through a peripheral vein. More ICG is needed in patients affected by emphysema to clearly visualize the intersegmental plain. NIR imaging starts immediately before the application of the contrast agent to gain a perfect dynamic and well-contrasted view of the boundaries between vascularized and non-vascularized lung segments. Immediately after ICG injection, we administer a 10-mL bolus of saline solution. In about 30–40 seconds after injection and during NIR visualization, the segment that needs to be resected appears “light grey” while the rest of the lung “switches on” in blue/green (Figure 3). Maximum green intensity is gained in about 1 min. The limits of the segment to be resected are marked by electrocautery during NIR visualization.
Case series and personal experience
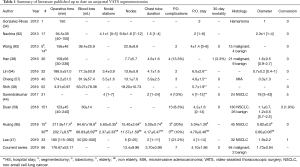
Data from principal studies on U-VATS segmentectomies are summarized in Table 1.
Full table
In 2013, Gonzalez-Rivas (96) reported his pionieristic experience on 17 U-VATS anatomic segmentectomies. Lingulectomy represented the most frequent segmentectomy. The mean surgical time was 94.5±35 minutes (40–150 minutes). The mean number of nodal stations explored was 4.1±1 (range, 0–5) with a mean of 9.6±1.8 (range, 7–12) lymph node resections. The median tumor size was 2.3±1 cm (range, 1–4 cm). The median chest tube duration was 1.5 days (range, 1–4 days) and the median length of hospital stay was 2 days (range, 1–6 days). He reported no conversion to MP-VATS or open surgery. Compared to thoracotomic segmentectomies, uniportal thoracoscopic segmentectomies were associated with a shorter length of hospital stay and with equivalent morbidity and mortality (97).
In 2016, Han (38) reported his experience on 30 U-VATS segmentectomies. A 3- to 4-cm intercostal surgical incision was performed in 16 patients, and a 2-cm intercostal incision in 14 patients. The most frequently removed segment was the upper left division (9 patients, 30%). The U-VATS segmentectomy was completed in all patients except for one (96.7%), who underwent lobectomy after the lesion was not found in the initially removed segment. The mean surgical time was 159±56 min, and it wasn’t influenced by the size of the surgical incision. The right middle and superior segments tended to require shorter operation times (97.1±44.9 min) than the other segments (P<0.001). The chest tube drainage was removed 4.6±1.6 days after the operation. One patient died (3.3%) during the post-surgical hospital stay because of septic shock.
Duan et al. (59) described their experience on 156 cases of U-VATS anatomical pulmonary segmentectomies performed between 2015 and 2016. They completed U-VATS pulmonary segmentectomies in 151 (96.8%) patients. Most cases involved the R1–2 and the left trisegment. Lung cancer was found in 130 cases and benign lesions in 26 cases. The comparison between operations performed in 2015 and 2016 showed that surgical time and blood loss were significantly lower during the second year (146 ± 56 vs. 113 ± 32 min and 63 ± 17 vs. 54 ± 13 mL respectively), so as complication rates (13.5% vs. 5.8%).
The role of U-VATS segmentectomy in elderly vs. non-elderly patients was addressed by Huang (95), who examined 139 elderly (124 lobectomies, 15 segmentecomies) and 278 non-elderly cases (248 lobectomies, 30 segmentectomies). Non statistical differences were found in between the two groups in segmentectomy population in terms of operative time, intraoperative blood loss, number of nodal stations explored and harvested lymph nodes. Elderly patients showed higher risk of postoperative complications, although without statistical significance (P=0.35).
Lin et al. (94) EP conducted a retrospective observational study comparing Uniportal VATS segmentectomies and lobectomies. They analysed 79 patients (32 segmentectomies, 47 lobectomies) affected by early stage NSCLC (T1a for segmentectomies, 7.3±2.4 mm; T1b/T1c for lobectomies 16.2±9.0 mm). In this study, no statistically significant differences between U-VATS anatomical segmentectomies and U-VATS lobectomies were found in terms of surgical time, intraoperative blood loss and number of staplers used. On the contrary, there was a statistically significant difference between the number of lymph nodes removed (13.6±5.8 for segmentectomies vs. 16.7±6.2 for lobectomies) and the duration of drainage stay (4.7±1.6 for segmentectomies vs. 5.9±3.2 for lobectomies). In this experience, segmentectomies seemed to allow a more rapid post-operative recovery. However, in the conclusions it became clear that they represent a technically more difficult procedure than lobectomies and therefore must be performed only by surgeons who are highly specialized in anatomic pulmonary resections with minimally invasive techniques.
Our experience
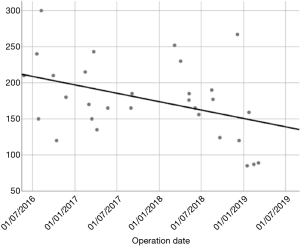
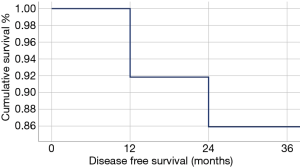
In our Department of General Thoracic Surgery at Policlinico Universitario “A.Gemelli” IRCCS in Rome, Italy, 66 Uniportal VATS segmentectomies were performed between May 2016 and August 2019. Our surgical technique is widely described elsewere (92). The mean age of patients was 65.70±12.52 years, 43.9% (29) of the patients were male and 34.8% (23 patients) were active smokers. The principal clinicopathological characteristics of the patients are reported in Table 2. The main indication to an anatomical segmentectomy was a primary lung cancer or a pulmonary metastasis. The mean diameter of the lesions was 1.73±0.94 cm at pathological examination with a mean of 13.40±9.96 lymph-nodes sampled (Table 2). In 45 (68.2%) patients the diagnosis was a primary lung cancer, in 19 (28.8%) a pulmonary metastasis and in 2 cases (3.0%) a benign lesion with [18F]-fluorodeoxyglucose (FDG) up-take at the preoperative positron emission tomography/computed tomography (PET/CT). The mean operative time was 176.67±53.17 minutes and it followed a trend of reduction during our experience, well represented by the inverse linear function: y= −kx + q (Pearson’s correlation Coefficient k=−0.405, P=0.027), Figure 4. There was no conversion to other surgical approaches and intraoperative mortality was null. The incidence of post-operative complications was 10.66% (7 cases). All complications were minor, like air-leakage (4 cases), pneumonia (2 cases) and atrial fibrillation (1 case). The mean chest-tube duration was 3.70±0.99 days, with a post-operative hospital stay of 4.10±1.86 days. Thirty-days mortality was null. All resections were radical. Only 3 (4.54%) patients underwent adjuvant therapy. At a mean follow-up of 18.36±10.55 months, 2 (3.03%) patients were alive with metastatic disease and 1 (1.52%) dead because of progression of the disease (Table 3). The mean disease-free survival was 17.42±10.54 months (92% at 1 year, 86% at 2 years) (Figure 5).
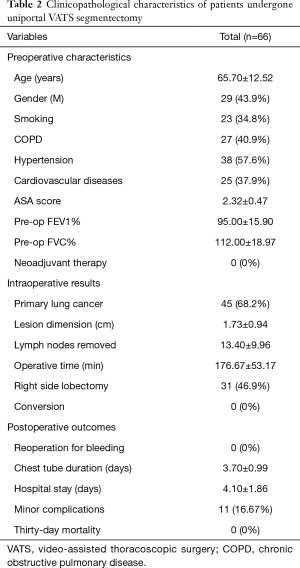
Full table
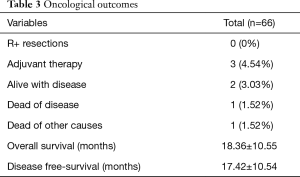
Full table
Conclusions
U-VATS segmentectomy is a technically feasible and safe procedure, with a reasonable learning curve and low percentage of conversion in multiportal or open procedures. Perioperative results show benefits in terms of width of the surgical wound, postoperative pain and faster recovery after U-VATS segmentectomy when compared with multiportal or open approaches. To the best of our knowledge, no other studies report long term results. More data are needed to definitively establish the role of segmentectomy and in particular U-VATS segmentectomy in the treatment of early NSCLC.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Mario Nosotti, Ilaria Righi and Lorenzo Rosso) for the series “Early Stage Lung Cancer: Sublobar Resections are a Choice?” published in Journal of Thoracic Disease. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.01.12). The series “Early Stage Lung Cancer: Sublobar Resections are a Choice?” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and non-randomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Travis WD, Asamura H, Bankier AA, et al. The IASLC lung cancer staging project: proposals for coding T categories for subsolid nodules and assessment of tumor size in part-solid tumors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2016;11:1204-23.
- Okada M, Nishio W, Sakamoto T, et al. Effect of tumor size on prognosis in patients with non-small cell lung cancer: the role of segmentectomy as a type of lesser resection. J Thorac Cardiovasc Surg 2005;129:87-93. [Crossref] [PubMed]
- Bedetti B, Bertolaccini L, Rocco R, et al. Segmentectomy versus lobectomy for stage I non-small cell lung cancer: a systematic review and meta-analysis. J Thorac Dis 2017;9:1615-23. [Crossref] [PubMed]
- Mun M, Kohno T. Efficacy of thoracoscopic resection for multifocal bronchioloalveolar carcinoma showing pure ground-glass opacities of 20 mm or less in diameter. J Thorac Cardiovasc Surg. 2007;134:877-82. [Crossref] [PubMed]
- González-Rivas D, Lirio F, Sesma J. Uniportal anatomic combined unusual segmentectomies. J Vis Surg 2017;3:91. [Crossref] [PubMed]
- Cao C, Gupta S, Chandrakumar D, et al. A critical analysis of segmentectomy versus lobectomy for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;46:928-9. [Crossref] [PubMed]
- Ghaly G, Kamel M, Nasar A, et al. Video-Assisted thoracoscopic surgery is a safe and effective alternative to thoracotomy for anatomical segmentectomy in patients with clinical stage I non-small cell lung cancer. Ann Thorac Surg 2016;101:465-72. [Crossref] [PubMed]
- Lex JR, Naidu B. In patients with resectable non-small-cell lung cancer, is video-assisted thoracoscopic segmentectomy an appropriate alternative to video-assisted thoracoscopic lobectomy? Interact Cardiovasc Thorac Surg 2016;23:826-31. [Crossref] [PubMed]
- Witte B, Stenz C, Vahl CF, et al. Comparative intention-to-treat analysis of the video-assisted thoracoscopic surgery approach to pulmonary segmentectomy for lung carcinoma. Interact Cardiovasc Thorac Surg 2015;21:276-83. [Crossref] [PubMed]
- Leshnower BG, Miller DL, Fernandez FG, et al. Video-assisted thoracoscopic surgery segmentectomy: a safe and effective procedure. Ann Thorac Surg 2010;89:1571-6. [Crossref] [PubMed]
- Yamashita S, Tokuishi K, Anami K, et al. Thoracoscopic segmentectomy for T1 classification of non-small cell lung cancer: a single center experience. Eur J Cardiothorac Surg 2012;42:83-8. [Crossref] [PubMed]
- Hwang Y, Kang CH, Kim HS, et al. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy on the patients with non-small cell lung cancer: a propensity score matching study. Eur J Cardiothorac Surg 2015;48:273-8. [Crossref] [PubMed]
- Watanabe A, Ohori S, Nakashima S, et al. Feasibility of video-assisted thoracoscopic surgery segmentectomy for selected peripheral lung carcinomas. Eur J Cardiothorac Surg 2009;35:775-80; discussion 780. [Crossref] [PubMed]
- Schuchert MJ, Pettiford BL, Pennathur A, et al. Anatomic segmentectomy for stage I non-small-cell lung cancer: comparison of video-assisted thoracic surgery versus open approach. J Thorac Cardiovasc Surg 2009;138:1318-25.e1. [Crossref] [PubMed]
- Sugi K, Kobayashi S, Sudou M, et al. Long-term prognosis of video-assisted limited surgery for early lung cancer. Eur J Cardiothorac Surg 2010;37:456-60. [PubMed]
- Witte B, Wolf M, Hillebrand H, et al. Complete video- assisted thoracoscopic surgery anatomic segmentectomy for clinical stage I lung carcinoma—technique and feasibility. Interact Cardiovasc Thorac Surg 2011;13:148-52. [Crossref] [PubMed]
- Zhong C, Fang W, Mao T, et al. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy for small-sized stage IA lung cancer. Ann Thorac Surg 2012;94:362-7. [Crossref] [PubMed]
- Tsutani Y, Miyata Y, Nakayama H, et al. Appropriate sublobar resection choice for ground glass opacity-dominant clinical stage IA lung adenocarcinoma: wedge resection or segmentectomy. Chest 2014;145:66-71. [Crossref] [PubMed]
- Cao J, Yuan P, Wang Y, et al. Survival Rates After Lobectomy, Segmentectomy, and Wedge Resection for Non-Small Cell Lung Cancer. Ann Thorac Surg 2018;105:1483-91. [Crossref] [PubMed]
- Huang Q, Wang R, Gu C, et al. Appropriate lymphadenectomy significantly reduced recurrence after segmentectomy for patients with non-small cell lung cancer. J Thorac Dis 2018;10:1919-26. [Crossref] [PubMed]
- Khullar OV, Liu Y, Gillespie T, et al. Survival After Sublobar Resection versus Lobectomy for Clinical Stage IA Lung Cancer: An Analysis from the National Cancer Data Base. J Thorac Oncol 2015;10:1625-33. [Crossref] [PubMed]
- Subramanian M, McMurry T, Meyers BF, et al. Long-Term Results for Clinical Stage IA Lung Cancer-Comparing Lobectomy and Sublobar Resection. Ann Thorac Surg 2018;106:375-81. [Crossref] [PubMed]
- Yim AP. VATS major pulmonary resection revisited- controversies, techniques, and results. Ann Thorac Surg 2002;74:615-23. [Crossref] [PubMed]
- Carr S, Schuchert M, Pennathur A, et al. Impact of Tumor Size on Outcomes After Anatomic Lung Resection for Stage 1A Non-Small Cell Lung Cancer Based on the Current Staging System. J Thorac Cardiovasc Surg 2012;143:390-7. [Crossref] [PubMed]
- Lopez-Pastorini A, Koryllos A, Schnell J, et al. Perioperative Outcome After Open and Thoracoscopic Segmentectomy for the Treatment of Malignant and Benign Pulmonary Lesions: A Propensity-Matched Analysis. J Thorac Dis 2018;10:3651-60. [Crossref] [PubMed]
- Atkins BZ, Harpole DH Jr, Mangum JH, et al. Pulmonary segmentectomy by thoracotomy or thoracoscopy: reduced hospital length of stay with a minimally-invasive approach. Ann Thorac Surg 2007;84:1107-12; discussion 1112-3. [Crossref] [PubMed]
- Yang CF, D’Amico TA. Thoracoscopic segmentectomy for lung cancer. Ann Thorac Surg 2012;94:668-81. [Crossref] [PubMed]
- Shiraishi T, Shirakusa T, Iwasaki A, et al. Video-assisted thoracoscopic surgery (VATS) segmentectomy for small peripheral lung cancer tumors: intermediate results. Surg Endosc 2004;18:1657-62. [PubMed]
- Gonzalez-Rivas D, Fieira E, Mendez L, et al. Single-port video-assisted thoracoscopic anatomic segmentectomy and right upper lobectomy. Eur J Cardiothorac Surg 2012;42:e169-71. [Crossref] [PubMed]
- Hernandez-Arenas LA, Lin L, Yang Y, et al. Initial experience in uniportal subxiphoid video-assisted thoracoscopic surgery for major lung resections. Eur J Cardiothorac Surg 2016;50:1060-6. [Crossref] [PubMed]
- Andrade H, Joubert P, Vieira A, et al. Single-port right upper lobe sleeve lobectomy for a typical carcinoid tumour. Interact Cardiovasc Thorac Surg 2017;24:315-6. [PubMed]
- Gonzalez-Rivas D, Fernandez R, Fieira E, et al. Uniportal video-assisted thoracoscopic bronchial sleeve lobectomy: first report. J Thorac Cardiovasc Surg 2013;145:1676-7. [Crossref] [PubMed]
- Han KN, Kim HK, Lee HJ, et al. Single-port video-assisted thoracoscopic pulmonary segmentectomy: a report on 30 cases. Eur J Cardiothorac Surg 2016;49:i42-7. [PubMed]
- Shih CS, Liu CC, Liu ZY, et al. Comparing the post- operative outcomes of video-assisted thoracoscopic surgery (VATS) segmentectomy using a multi-port technique versus a single-port technique for primary lung cancer. J Thorac Dis 2016;8:S287-94. [PubMed]
- Gonzalez-Rivas D, Yang Y, Stupnik T, et al. Uniportal video-assisted thoracoscopic bronchovascular, tracheal and carinal sleeve resections. Eur J Cardiothorac Surg 2016;49:i6-16. [PubMed]
- Gonzalez-Rivas D, Delgado M, Fieira E, et al. Single-port video-assisted thoracoscopic lobectomy with pulmonary artery reconstruction. Interact Cardiovasc Thorac Surg 2013;17:889-91. [Crossref] [PubMed]
- Jutley RS, Khalil MW, Rocco G. Uniportal vs. standard three-port VATS technique for spontaneous pneumothorax: comparison of post-operative pain and residual paraesthesia. Eur J Cardiothorac Surg 2005;28:43-6. [Crossref] [PubMed]
- Harris CG, James RS, Tian DH, et al. Systematic review and meta-analysis of uniportal versus multiportal video-assisted thoracoscopic lobectomy for lung cancer. Ann Cardiothorac Surg 2016;5:76-84. [Crossref] [PubMed]
- Surendrakumar V, Martin-Ucar AE, Edwards JG, et al. Evaluation of surgical approaches to anatomical segmentectomies: the transition to minimal invasive surgery improves hospital outcomes. J Thorac Dis 2017;9:3896-902. [Crossref] [PubMed]
- Ji C, Xiang Y, Pagliarulo V, et al. A multi-center retrospective study of single-port versus multi-port video-assisted thoracoscopic lobectomy and anatomic segmentectomy. J Thorac Dis 2017;9:3711-8. [Crossref] [PubMed]
- Han KN, Kim HK, Choi YH, et al. Comparison of single port versus multiport thoracoscopic Segmentectomy. J Thorac Dis 2016;8:S279-86. [PubMed]
- Lee J, Lee JY, Choi JS, et al. Comparison of Uniportal versus Multiportal Video-Assisted Thoracoscopic Surgery Pulmonary Segmentectomy. Korean J Thorac Cardiovasc Surg 2019;52:141-7. [Crossref] [PubMed]
- Wang BY, Liu CY, Hsu PK, et al. Single-incision versus multiple-incision thoracoscopic lobectomy and segmentectomy: a propensity-matched analysis. Ann Surg 2015;261:793-9. [Crossref] [PubMed]
- Chen YY, Lin TH, Chang CC, et al. Comparison of uniportal and multiportal video-assisted thoracoscopic (VATS) complicated segmentectomy: a propensity score matching analysis. J Thorac Dis 2017;9:AB009. [Crossref]
- Ceppa DP, Balderson S, D’Amico TA. Technique of thoraco-scopic basilar segmentectomy. Semin Thorac Cardiovasc Surg 2011;23:64-6. [Crossref] [PubMed]
- Nomori H, Okada M. Illustrated anatomical segmentectomy for lung cancer. Tokyo: Springer, 2012.
- Endoh M, Oizumi H, Kato H, et al. Posterior approach to thoracoscopic pulmonary segmentectomy of the dorsal basal segment: a single-institute retrospective review. J Thorac Cardiovasc Surg 2017;154:1432-9. [Crossref] [PubMed]
- Hernandez-Arenas LA, Purmessur RD, Gonzalez-Rivas D. Uniportal video-assisted thoracoscopic segmentectomy. J Thorac Dis 2018;10:S1205-14. [Crossref] [PubMed]
- McKenna RJ Jr. Complications and learning curves for video-assisted thoracic surgery lobectomy. Thorac Surg Clin 2008;18:275-80. [Crossref] [PubMed]
- Petersen RH, Hansen HJ. Learning curve associated with VATS lobectomy. Ann Cardiothorac Surg 2012;1:47-50. [PubMed]
- Hernandez-Arenas LA, Lin L, Purmessur RD, et al. Uniportal video-assisted thoracoscopic early learning curve for major lung resections in a high volume training center. J Thorac Dis 2018;10:S3670-7. [Crossref] [PubMed]
- Cheng K, Zheng B, Zhang S, et al. Feasibility and learning curve of uniportal video-assisted thoracoscopic segmentectomy. J Thorac Dis 2016;8:S229-34. [PubMed]
- Nachira D, Meacci E, Porziella V, et al. Learning curve of uniportal Video assisted lobectomy: analysis of 15-months experience in a single center. J Thorac Dis 2018;10:S3662-9. [Crossref] [PubMed]
- Duan L, Jiang G, Yang Y. One hundred and fifty-six cases of anatomical pulmonary segmentectomy by uniportal video-assisted thoracic surgery: a 2-year learning experience. Eur J Cardiothorac Surg 2018;54:677-82. [Crossref] [PubMed]
- Okada M, Mimura T, Ikegaki J, et al. A novel video-assisted anatomic segmentectomy technique: selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg 2007;133:753-8. [Crossref] [PubMed]
- Schuchert MJ, Abbas G, Awais O, et al. Anatomic segmentectomy for the solitary pulmonary nodule and early-stage lung cancer. Ann Thorac Surg 2012;93:1780-5. [Crossref] [PubMed]
- Wada H, Anayama T, Hirohashi K, et al. Thoracoscopic ultrasonography for localization of subcentimetre lung nodules. Eur J Cardiothorac Surg 2016;49:690-7. [Crossref] [PubMed]
- Chen S, Zhou J, et al. Video-assisted thoracoscopic solitary pulmonary nodule resection after CT-guided hookwire localization: 43 cases report and literature review. Surg Endosc 2011;25:1723-9. [Crossref] [PubMed]
- Kim HK, Han KN. Uniportal Video-Assisted Thoracoscopic Surgery Segmentectomy. Thorac Surg Clin 2017;27:387-98. [Crossref] [PubMed]
- Doo KW, Yong HS, Kim HK, et al. Needlescopic resection of small and superficial pulmonary nodule after computed tomographic fluoroscopy-guided dual localization with radiotracer and hookwire. Ann Surg Oncol 2015;22:331-7. [Crossref] [PubMed]
- McWilliams A, Shaipanich T, Lam S. Fluorescence and navigational bronchoscopy. Thorac Surg Clin 2013;23:153-61. [Crossref] [PubMed]
- Kalanjeri S, Gildea TR. Electromagnetic navigational bronchoscopy for peripheral pulmonary nodules. Thorac Surg Clin 2016;26:203-13. [Crossref] [PubMed]
- Yu PS, Chu CM, Lau RWH, et al. Video-assisted thoracic surgery for tiny pulmonary nodules with real-time image guidance in the hybrid theatre: the initial experience. J Thorac Dis 2018;10:2933-9. [Crossref] [PubMed]
- Brown J, Lee TJ, Joiner T, et al. Using electromagnetic navigation bronchoscopy and dye injection to aid in video-assisted lung resection. Am Surg 2016;82:1052-4. [Crossref] [PubMed]
- Nagai K, Kuriyama K, Inoue A, et al. Computed tomography-guided preoperative localization of small lung nodules with indocyanine green. Acta Radiologica 2018;59:830-5. [Crossref] [PubMed]
- Findik G, Demiröz SM, Apaydın SMK, et al. Computed tomography-guided methylene blue labeling prior to thoracoscopic resection of small deeply placed pulmonary nodules. Do we really need palpation? Thorac Cardiovasc Surg 2017;65:387-91. [Crossref] [PubMed]
- Lin MW, Tseng YH, Lee YF, et al. Computed tomography-guided patent blue vital dye localization of pulmonary nodules in uniportal thoracoscopy. J Thorac Cardiovasc Surg 2016;152:535-44.e2. [Crossref] [PubMed]
- Galetta D, Bellomi M, Grana C, et al. Radio-guided localization and resection of small or ill-defined pulmonary lesions. Ann Thorac Surg 2015;100:1175-80. [Crossref] [PubMed]
- Mogi A, Yajima T, Tomizawa K, et al. Video-assisted thoracoscopic surgery after preoperative CT-guided lipiodol marking of small or impalpable pulmonary nodules. Ann Thorac Cardiovasc Surg 2015;21:435-9. [Crossref] [PubMed]
- Lee NK, Park CM, Kang CH, et al. CT-guided percutaneous transthoracic localization of pulmonary nodules prior to video-assisted thoracoscopic surgery using barium suspension. Korean J Radiol 2012;13:694-701. [Crossref] [PubMed]
- Thaete FL, Peterson MS, Plunkett MB, et al. Computed tomography-guided wire localization of pulmonary lesions before thoracoscopic resection: results in 101 cases. J Thorac Imaging 1999;14:90-8. [Crossref] [PubMed]
- Congedo MT, Iezzi R, Nachira D, et al. Uniportal VATS Coil-Assisted Resections for GGOs. J Oncol 2019;2019:5383086. [Crossref] [PubMed]
- Sui X, Zhao H, Yang F, et al. Computed tomography guided microcoil localization for pulmonary small nodules and ground-glass opacity prior to thoracoscopic resection. J Thorac Dis 2015;7:1580-7. [PubMed]
- Ma M, He F, Lv X, et al. Feasibility and Effectiveness of Thoracoscopic Pulmonary Segmentectomy for Non-Small Cell Lung Cancer. Medicine (Baltimore) 2020;99:e18959. [Crossref] [PubMed]
- Kamiyoshihara M, Kakegawa S, Morishita Y. Convenient and improved method to distinguish the intersegmental plane in pulmonary segmentectomy using a butterfly needle. Ann Thorac Surg 2007;83:1913-4. [Crossref] [PubMed]
- Oizumi H, Kato H, Endoh M, et al. Slip knot bronchial ligation method for thoracoscopic lung segmentectomy. Ann Thorac Surg 2014;97:1456-8. [Crossref] [PubMed]
- Sugimoto S, Oto T, Miyoshi K, et al. A novel technique for identification of the lung intersegmental plane using dye injection into the segmental pulmonary artery. J Thorac Cardiovasc Surg 2011;141:1325-7. [Crossref] [PubMed]
- Oh S, Suzuki K, Miyasaka Y, et al. New Technique for Lung Segmentectomy Using Indocyanine Green Injection. Ann Thorac Surg 2013;95:2188-90. [Crossref] [PubMed]
- Sekine Y, Ko E, Oishi H, et al. A simple and effective technique for identification of intersegmental planes by infrared thoracoscopy after transbronchial injection of indocyanine green. J Thorac Cardiovasc Surg 2012;143:1330-5. [Crossref] [PubMed]
- Kato H, Oizumi H, Suzuki J, et al. Thoracoscopic anatomical lung segmentectomy using 3D computed tomography simulation without tumour markings for non-palpable and non-visualized small lung nodules. Interact Cardiovasc Thorac Surg 2017;25:434-41. [Crossref] [PubMed]
- Pardolesi A, Veronesi G, Solli P, et al. Use of indocyanine green to facilitate intersegmental plane identification during robotic anatomic segmentectomy. J Thorac Cardiovasc Surg 2014;148:737-8. [Crossref] [PubMed]
- Sato M, Murayama T, Nakajima J. Techniques of stapler-based navigational thoracoscopic segmentectomy using virtual assisted lung mapping (VAL-MAP). J Thorac Dis 2016;8:S716-30. [Crossref] [PubMed]
- Misaki N, Chang SS, Gotoh M, et al. A novel method for determining adjacent lung segments with infrared thoracoscopy. J Thorac Cardiovasc Surg 2009;138:613-8. [Crossref] [PubMed]
- Misaki N, Chang SS, Igai H, et al. New clinically applicable method for visualizing adjacent lung segments using an infrared thoracoscopy system. J Thorac Cardiovasc Surg 2010;140:752-6. [Crossref] [PubMed]
- Tarumi S, Misaki N, Kasai Y, et al. Clinical trial of video-assisted thoracoscopic segmentectomy using infrared thoracoscopy with indocyanine green. Eur J Cardiothorac Surg 2014;46:112-5. [Crossref] [PubMed]
- Meacci E, Nachira D, Congedo MT, et al. Uniportal video-assisted thoracic lung segmentectomy with near infrared/indocyanine green intersegmental plane identification. J Vis Surg 2018;4:17. [Crossref] [PubMed]
- Nachira D, Meacci E, Petracca Ciavarella L, et al. Uniportal video-assisted thoracic surgery Roman experience-a report of the first 16-month Roman experience. J Thorac Dis 2018;10:S3678-85. [Crossref] [PubMed]
- Wang BY, Tu CC, Liu CY, et al. Single-Incision Thoracoscopic Lobectomy and Segmentectomy With Radical Lymph Node Dissection. Ann Thorac Surg 2013;96:977-82. [Crossref] [PubMed]
- Lin Y, Zheng W, Zhu Y, et al. Comparison of treatment outcomes between single-port video-assisted-thoracoscopic anatomic segmentectomy and lobectomy for non-small celllung cancer of early-stage: a retrospective observational study. J Thorac Dis 2016;8:1290-6. [Crossref] [PubMed]
- Huang L, Zheng B, Chen C, et al. To Explore Clinical Value of Single-port Video-assisted Thoracoscopic Surgery in Elderly Patients with Non-small Cell Lung Cancer: Lobectomy, Segmentectomy and Lobectomy vs. Segmentectomy. Zhongguo Fei Ai Za Zhi 2018;21:287-95. [PubMed]
- Gonzalez-Rivas D, Mendez L, Delgado M, et al. Uniportal video-assisted thoracoscopic anatomic segmentectomy J Thorac Dis 2013;5:S226-33. [PubMed]
- Linden D, Linden K, Oparka J. In patients with resectable non-small-cell lung cancer, is video-assisted thoracoscopic segmentectomy a suitable alternative to thoracotomy and segmentectomy in terms of morbidity and equivalence of resection? Interact Cardiovasc Thorac Surg 2014;19:107-10. [Crossref] [PubMed]