Surgical options in atrial fibrillation
Atrial fibrillation (AF) is not benign. It affects more than 2.2 million Americans (1,2). This prevalence is associated with significant negative impact on quality of life, morbidity and mortality even with the rate and/or rhythm controlled (3-5). Problems related to tachycardia-induced cardiomyopathy, palpitation and/or heart failure are common among AF patients. Even in asymptomatic patients, the risks of stroke and systemic thromboembolism (STE) are serious threats. Out of every five all-cause strokes, 1 is secondary to AF (6). Those strokes related to AF also seem to be the more clinically significant and disabling ones (6,7). Moreover, patients with AF live with an increased procedural morbidity and mortality risk, secondary to hemodynamic effects and/or anticoagulation (AC), especially with emergency procedures.
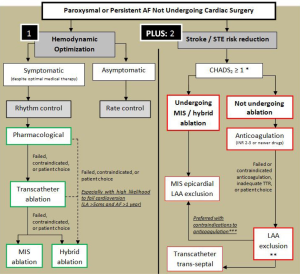
Management of AF is aimed at alleviating symptoms and two other main goals: (I) optimizing cardiac output through rhythm control or rhythm control; (II) decreasing the risk of cerebral and STE, with a minimal increase in the risk of intra and extra-cranial bleeding. Achieving these two goals can be less than straight forward. A simplified strategy for management is illustrated in Figure 1. Unfortunately, several studies have shown that even when resumption of sinus rhythm (SR) is possible, patients are still at an increased risk of stroke and STE (8,9). It is thus currently recommended to prescribe a modality for stroke risk reduction based on stroke risk assessment, commonly via the CHADS2 or CHADS-VASC score, regardless of the rhythm attained.
The body of literature is expanding and evolving, providing us with more informed decisions as to the best strategy for every specific patient. In this article, we will discuss the surgical strategies proposed to address the two main goals in the management of AF.
Surgical optimization of cardiac output
Generally, the decision whether to control rhythm or rate has been extensively studied. Results show that superiority of either strategy over the other is minimal and inconsistent (8-12). Rhythm control is more difficult to achieve with increasing age and left atrial size (13,14). Also, the duration of AF is directly proportional to the rates of failed cardioversion, suggesting that AF begets AF, probably through structural or conductance alterations of myocardial substrates over time (15). Rate control alone is usually preferred with asymptomatic chronic AF patients especially those older than 65 years, partly to simplify their pharmaceutical management, given that these patients are usually already on a number of other medications, and that antiarrythmic drugs (AAD) have significant drug interactions.
Surgical strategies for optimizing cardiac output in AF patients are largely rhythm controlling. An exception is the Corridor procedure; a rate controlling procedure that has not been widely adopted. This procedure isolates a strip of atrial myocardium that connects the sinoatrial node (SAN) to the atrioventricular node (AVN) (16,17). Eccentric triggers continue to fire, and the atria continue to fibrillate, but the AVN only receives triggers from the SAN through the strip. As such, the ventricular rate is controlled.
According to the consensus statement from the Heart Rhythm Society, endorsed by the European Heart Rhythm Society, the European Cardiac Arrhythmia Society, the American College of Cardiology (ACC), the American Heart Association (AHA) and the Society of Thoracic Surgeons (STS), rhythm controlling surgeries are indicated with symptomatic or selected asymptomatic AF patients undergoing cardiac surgery, or symptomatic lone AF refractory to medical management, or after failed catheter ablation (18). The majority of AF surgeries are currently done in the context of concomitant cardiac surgery. However, stand-alone minimally invasive ablation or hybrid ablation procedures (combined surgical and catheter-based ablation) have seen increased adoption with improved outcomes.
The benchmark AF surgery is the Cox-Maze (CM) procedure, serially published by Cox and colleagues during the late 1980s and early 1990s (19-22). The procedure interrupts, by cutting and sewing, all potential myocardial substrates for re-entrance and AF signal propagation, while creating a “Maze” of functioning atrial myocardium, through which normal impulses can travel from the SAN to the AVN through both atria. The initial procedure was effective but was associated with significant chronotropic incompetence and high rates of pacemaker implantations (23). Serial modifications to address these issues and to technically simplify the procedure have been developed, culminating in the CM-III procedure (24). Other authors have proposed an alternative set of radial lesions diverging from the SAN, which seemed to be easier and as effective as the Maze procedure (25). The technique was however studied on a small number of patients and was not widely adopted. The CM-III did prove to be very effective. Cox and colleagues reported success rates approaching 95% after 10 years follow-up (26). Others too have reported similar results (27-30). Adding to rhythm control, Cox and colleagues reported their 11.5-year follow up of 306 AF patients who underwent a Maze procedure, 58 of whom had a history of a cerebrovascular accident. Seventy-two percent of the patients were not receiving AC during the follow up period. The authors reported a single minor stroke in all patients over the period of follow up (31). Despite these results, there is no consistency among the literature, nor there is an agreement that rhythm control, regardless the method used, would offer a stroke risk reduction that would allow for safe discontinuation of AC or the refrain from other stroke risk reduction strategies.
A major criticism of the CM-III that hindered its wide spread use was one of complexity and technical difficulty. Alternating bradyarrythmias and tachyarrythmias are other problems that have been described especially with exercise and other forms of stress. These symptoms however improve with autonomic re-innervation over time (32). Moreover, with extensive atrial scarring, restoration of synchronized atrial contraction does not necessarily lead to restoration of atrial functional contraction, and may not help much in patients with ventricular diastolic dysfunction (33). These issues seem to be true for most ‘cut-and-sew’ techniques in general and thus are less commonly adopted in recent years. Another criticism of all ablation strategies on an arrested heart, is the inability to identify the exact propagation pathways in specific patients, which thus necessitates a rather extensive full set of empiric lesions to ensure interruption of any potential substrate.
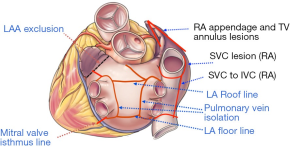
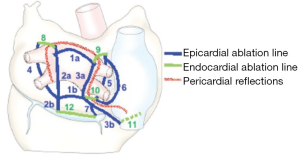
With the advent of energy-based ablation devices to replace the original cut-and-sew technique, the CM-IV was described (34,35). Myocardial ablation is done using a combination of bipolar radiofrequency and cryoablation. Cryoablation is generally less deforming to the cardiac fibrous skeleton, and is thus used near valvular annuli and trigones. The procedure is performed using cardiopulmonary bypass (CPB) either through a sternotomy for concomitant surgery or through a right mini-thoracotomy for lone AF, and the lesions are similar to those of the CM-III (Figure 2). Typically, right atrial lesions can be done on CPB and a beating heart to decrease clamp and ischemic time.
The goal for which the CM-IV was developed was achieved. The procedure is simpler, and clamp times for concomitant surgeries were reduced from a mean of 122 minutes with CM-III to 92 minutes with CM-IV, and for lone AF from a mean of 93 minutes with CM-III to 41 minutes with CM-IV (34,36). The CM-IV proved to be successful in regaining SR, but not as efficacious as CM-III. A study of 100 lone AF patients undergoing CM-IV reported an 84% freedom of AF and AAD at 2 years (36). Another study of 282 AF patients CM-IV with concomitant cardiac surgery reported a 78% freedom of AF and AAD at 1 year (14). Although these numbers seem to be lower than those reported for CM-III at longer follow-ups, one can argue that patients were significantly different, and definitions for success of rhythm control were not standardized in most the CM-III reports. A propensity score matching comparing the two procedures showed similar rates of freedom from AF at 1 year (37). Interestingly though, the study did not report any decrease in morbidity with CM-IV compared to CM-III.
Less extensive lesion sets have been adopted by some to simplify the procedure. Haïssaguerre and colleagues described the pulmonary veins as the source of ectopia in most AF (38). Different lesion used for pulmonary vein isolation (PVI) include individual vein isolation, right and left veins isolation with or without connecting lesions or isolation of the whole left atrial posterior wall via a box-like lesion. An attractive aspect to PVI is that it can be done off CPB. A small randomized controlled trial (RCT) of 30 patients undergoing mitral valve surgery alone or with either a cut-sew Maze or PVI showed PVI to be as effective as Maze with mitral valve surgery (39). Results from other small studies however seem to be heterogeneous and inconsistent, reporting a 1 year freedom from AF and AAD ranging from 20-88% (40-43). One study reported only 17% freedom from AF and AAF at a mean of 31 months follow up after cryoablation PVI with concomitant cardiac surgery (44). This raises questions about the transmurality and durability of a solely epicardial procedure done off-pump. Generally, we regard PVI to be simpler but less efficacious than CM-IV. The fact that it can be done off-pump puts it in comparison with transcatheter ablation (TCA). The FAST trial randomized 124 refractory AF patients to TCA or minimally invasive (MIS) PVI with optional additional lesions at the discretion of the surgeon (45). Left atrial dimensions and pressures were elevated in 33% of patients, and 67% had a failed prior TCA. Freedom from AF and AAD at 1 year was 66% with MIS PVI versus 37% with TCA. The higher efficacy with MIS PVI was associated with a higher procedural adverse event rate (34% versus 16%). The results, although not very clear, suggest an equal of inferior efficacy compared to CM-III and IV, while possibly superior to TCA. Larger randomized studies however are warranted to better delineate the performance and role of PVI with concomitant surgery and in lone AF.
Left atrial lesions alone or added to PVI also have it proponents. Left atrial lesion sets vary by center and by surgeon and results are inconsistent. A number of studies suggest that bi-atrial approaches are more effective than isolated left atrial lesions, which is in agreement with the fact that some AF patients have unstable foci across both atria. A RCT is currently recruiting AF patients undergoing mitral valve surgery to left atrial lesions versus bi-atrial lesions versus no lesions at all. The study is estimated to enroll 260 patients and to publish their results by the end of 2014. As of now, we recommend bi-atrial ablation. A meta-analysis including 2,260 patients showed that bi-atrial ablation surgical procedures to be more effective in controlling AF than procedures confined to the left atrium (96% versus 89%, P<0.001) (46).
Addressing suboptimal transmurality of lesions achieved by purely endocardial TCA or purely epicardial MIS procedures, while still avoiding CPB and maintaining minimal invasiveness, hybrid procedures have been developed (Figure 3). These procedures have been described in either a one-stage setting or on sequential stages (47-51). Theoretical advantages related to the MIS aspect are avoidance of phrenic nerve and esophageal injuries as well as reduction of postoperative cardiac tamponade. These are related to surgeons’ familiarity with the anatomy of the chest and avoidance of injury, as well as opening the pericardium. Also, with decreasing endocardial lesions, one would expect a decreased rate of thrombus formation and embolism. These procedures take longer time compared to MIS procedures or TCA each alone, however, early results suggest a higher efficacy compared to either (48,52). The procedure can also be simplified to lesions that are necessary for every specific patient through electro-physiologic mapping and identification of patient specific substrates. We expect that studies will prove hybrid procedures to be comparable in efficacy and superior in safety compared to CM-IV in lone AF. A recent review published hybrid experience included 335 patients with lone AF from nine studies (53). Published studies are small in number and mostly single center experiences. The review described superiority of the bilateral approach, and although results are very promising, further larger studies are warranted. The multi-center Dual Epicardial Endocardial Persistent AF (Staged-DEEP) trial is ongoing, and estimated to conclude by the end of 2015. This can give us more data regarding the safety and efficacy of hybrid approaches.
The literature is heterogeneous with inconsistencies in the types of patients studied, the indications, the lesion sets, the energy devices used, and very importantly, the outcome definitions. This makes results difficult to interpret and comparing different strategies problematic. With the recent standardization of indications and definitions, the more recent studies are more informative, but still lack larger RCTs. We generally regard the CM-III to be the gold standard, to which other surgical and catheter-based procedures should be compared. RCTs comparing CM-III to other lesion sets and techniques would be optimal, however technical difficulty over CM-III would make comparisons to CM-IV instead an accepted and more feasible alternative for future research. Surgical interventions are generally more effective than TCA, but might come at a higher morbidity. A recent systemic review of recent studies comparing different surgical approaches to TCA demonstrated a general higher rate of freedom from AF at 1 year with surgical approaches (54). Morbidity rates were similar except for a higher rate of pacemaker implantations with surgery. Results from the FAST trial make similar conclusion, with MIS PVI (45). With concomitant surgery, we recommend a full CM-IV lesion set. With lone AF, the guidelines currently recommend surgical ablation as an option following failure of pharmacotherapy, TCA failure or at patient’s request. However, with the advent of hybrid approaches, we expect this to change soon, given the superior efficacy of MIS, adding to the more durable transmurality and procedural safety expected with endocardial and epicardial approaches combined. We also believe a change will take place in the belief that rhythm and rate control offer equal morbidity and mortality. Studies leading to this conclusion have mostly included patients who are rhythm controlled medically (10,12). Thus, the great majority of these patients continued on AAD maintenance as well as AC. This has definitely added both morbidity and poor quality of life. With wider adoption of surgical and hybrid strategies neither drug classes might be needed. These strategies offer high freedom rates from AAD, and with the recent results confirming the non-inferiority of left atrial appendage (LAA) exclusion to warfarin therapy, we believe that rhythm control would be superior to rhythm control in most situations (55).
Surgical stroke and STE risk reduction
A patient with AF has a 5% annual risk of stroke, a 5-fold increase compared to the general population (6,7). These strokes are mostly embolic in nature with the left atrium and LAA as the sources. For years and till today, the gold standard stroke risk reduction strategy is AC by warfarin to an INR range of 2-3. However, due to numerous concerns with warfarin AC, alternatives have been explored. Novel anticoagulants including dabigatran, rivaroxaban and apixaban seem to have comparable efficacy with potentially better safety profile. However still, the nature of AC inevitably carries a risk of bleeding. The observation that 90% of the thrombi found in non-valvular AF patients are in the LAA and 57% in valvular AF triggered a lot of interest (56). As a result of this observation, LAAs have been ligated or excised during cardiac surgery in AF patients by many surgeons, largely based on intuition and no clear evidence of efficacy in stroke risk reduction. Till recently, the results were inconsistent, and high rates of incomplete occlusions have hindered drawing a definite relationship between LAA and stroke. As a result, till today AC is the recommended first line stroke risk reduction in AF, and the guidelines recommend LAA exclusion only with surgical ablation of AF or in the context of mitral valve surgery (57). Recently however, the RCT PROTECT AF demonstrated for the first time the non-inferiority, and in their follow up publication the superiority of LAA exclusion over warfarin therapy using the Watchman percutaneous device (55,58). Despite this, FDA concerns over device safety have significantly delayed approval (to date) and commercial availability outside of a trial setting. With a clear relationship established between the LAA and stroke in AF patients, more devices and techniques are being developed for effective, complete and reproducible LAA exclusion during surgery and as lone procedures.
The first RCT was published by Healey and colleagues (59), and demonstrated no increase in morbidity with LAA excision during cardiac surgery. Although proved to be surgically safe, skepticism regarding value in stroke risk reduction made many surgeons not do it. A major problem was incomplete closure. Results by García-Fernández (60) augmented this skepticism, demonstrating that incomplete LAA exclusion carries a 12-fold increase in the risk of stroke compared to not excluding it at all. With this background, complete closure rates reported as low as 40% success were problematic (61). Reasons behind incomplete closures were analyzed and investigated. Stapler or scissor excisions showed superior results to endocardial closure, regarding completeness of closure as well as feasibility with off-pump surgery. Recently, complete occlusion rates have drastically improved, with more authors publishing encouraging data. Bakhtiary and colleagues (62) reported 100% complete closure rates using a Derra clamp and a double level suture on an arrested heart. The same rate was achieved on a beating heart by Ohtsuka and colleagues (63) in lone AF patients through a MIS thoracoscopic procedure. No major complications were encountered and the mean procedural time was reported to be only 32 minutes. These small series demonstrated the safety and feasibility of isolated MIS LAA exclusion.
Epicardial LAA exclusion devices are commercially available. These have the advantage of not having any foreign body-blood contact and potentially have less thrombosis, infection and embolization. The TigerPaw II System (Maquet Cardiovascular LLC) can be used in the context of concomitant cardiac surgery and is approved by the food and drug administration (FDA). It is composed of a delivery tool and a fastener that is made of linearly spaced connectors over-molded with soft silicone (Figure 4A). Fifty four patients followed up by trans-esophageal echocardiography (TEE) 3 months after surgery all had complete occlusions (64). The other two devices can be used both with other cardiac surgeries as well as lone procedures. The Lariat suture delivery system (SentreHeart, Palo Alto, CA) combines an epicardial with a transcatheter endocardial approach. A magnet tip is deliver trans-cather trans-septal to attach to another guidewire that is epicardially inserted (Figure 4B). A radio-opaque tie is guided down the wire and tightened at the base of the LAA. Although the suture delivery system is FDA approved for “tissue-approximation”, it is not approved specifically for LAA ligation. Bartus and colleagues (65) reported their experience with 89 AF patients who are poor candidates for AC. The procedure seemed to be feasible with complete LAA occlusion rate of 89% at 1 year. Concerns however were related to myocardial puncture related to the catheter, as well related to severe pericarditis. These concerns were reproduced by the American experience. Two studies including 20 and 27 patients reported myocardial punctures requiring further procedures (66,67). These two studies also reported three patients per each study presenting with severe post-procedural pericarditis, necessitating hospital stay and at an instance, coronary angiography. Although the idea of the Lariat is novel, we believe these events should trigger questioning whether the suture system needs to be modified before it can be approved for safety in this particular indication of LAA occlusion. Furthermore, the efficacy of completeness of LAA closure remains questionable on medium and long-term follow-up—requiring further investigation.
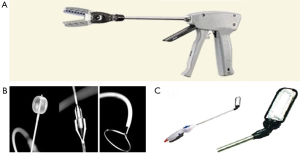
The Atriclip LAA exclusion system (Atricure Inc, Westchester, OH) has become, over the past few years, the preferred approach for safe and complete LAA exclusion in open cardiac surgery patients. It is approved for LAA exclusion during open cardiac surgery, and is currently being studied for MIS lone procedures. It is a self closing clip made of two parallel titanium tubes with elastic nitinol springs, covered by knit braided polyester (Figure 4C). The delivery system allows for re-deployment and repositioning; ensuring optimal placement at the base if placed suboptimally. Two studies on 34 and 71 patients undergoing cardiac surgery reported complete occlusion rates of 100% and 98% at three months respectively (68,69). There were no adverse events reported. Currently, a multi-center study for stand-alone LAA exclusion procedures using the AtriClip is recruiting patients with contraindications for AC and a CHADS2 score ≥2.
Well powered comparisons of these surgical devices to AC do not exist. The data however can be extrapolated from well powered RCT comparing percutaneous LAA occluding devices to warfarin. Now that LAA exclusion is proven to be at least a non-inferior alternative to warfarin therapy in non-valvular AF patients, the question should be directed to which device can achieve the most complete, reproducible closure through the safest procedure. Newer anticoagulants do address some of the disadvantages of warfarin, but do have their own shortcomings and the fact that the patient is still committed to lifelong AC and its associated morbidity. We believe that patients with AF undergoing cardiac surgery, should get their LAA excluded at a minimum. We expect future guidelines to allow for safe discontinuation of AC with TEE confirmation of complete LAA closure. MIS LAA exclusion (using AtriClip or other reliable devices) can be performed safely and leads to effective complete LAA closure.
With the availability of reliable occlusion devices, we recommend LAA exclusion in all AF patients undergoing cardiac surgery, regardless of their suitability for AC therapy. For lone AF patients with an embolic risk qualifying for AC, we recommend LAA exclusion in patients with failed AC or with relative or absolute contraindications to AC. We foresee that with expanding literature; demonstrating non-inferiority of effective LAA occlusion to AC, and with the rise of safe and effective occlusion devices, LAA exclusion will be also offered to patients who are good candidates for AC. We also believe the guidelines as well as patients’ choices will dictate whether a minimally invasive procedure would be more favourable than lifelong of AC with a bleeding risk that increases linearly over time.
Acknowledgements
Disclosure: Dr. Ramawi is Consultant, Advisory Board Member in AtriCure. Dr. Bedeir declares no conflict of interest.
References
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370-5. [PubMed]
- Thom T, Haase N, Rosamond W, et al. Heart disease and stroke statistics--2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2006;113:e85-151. [PubMed]
- Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation 2004;109:1509-13. [PubMed]
- Wyse DG, Love JC, Yao Q, et al. Atrial fibrillation: a risk factor for increased mortality--an AVID registry analysis. J Interv Card Electrophysiol 2001;5:267-73. [PubMed]
- Leong DP, Eikelboom JW, Healey JS, et al. Atrial fibrillation is associated with increased mortality: causation or association? Eur Heart J 2013;34:1027-30. [PubMed]
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983-8. [PubMed]
- Stöllberger C, Chnupa P, Abzieher C, et al. Mortality and rate of stroke or embolism in atrial fibrillation during long-term follow-up in the embolism in left atrial thrombi (ELAT) study. Clin Cardiol 2004;27:40-6. [PubMed]
- Van Gelder IC, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med 2002;347:1834-40. [PubMed]
- Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002;347:1825-33. [PubMed]
- Carlsson J, Miketic S, Windeler J, et al. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol 2003;41:1690-6. [PubMed]
- Opolski G, Torbicki A, Kosior DA, et al. Rate control vs rhythm control in patients with nonvalvular persistent atrial fibrillation: the results of the Polish How to Treat Chronic Atrial Fibrillation (HOT CAFE) Study. Chest 2004;126:476-86. [PubMed]
- Hohnloser SH, Kuck KH, Lilienthal J. Rhythm or rate control in atrial fibrillation--Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomised trial. Lancet 2000;356:1789-94. [PubMed]
- Ad N. The quest to identify predictors for success and failure after the Cox-Maze procedure for the treatment of atrial fibrillation. J Thorac Cardiovasc Surg 2010;139:117-8. [PubMed]
- Damiano RJ Jr, Schwartz FH, Bailey MS, et al. The Cox maze IV procedure: predictors of late recurrence. J Thorac Cardiovasc Surg 2011;141:113-21. [PubMed]
- Wijffels MC, Kirchhof CJ, Dorland R, et al. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995;92:1954-68. [PubMed]
- Leitch JW, Klein G, Yee R, et al. Sinus node-atrioventricular node isolation: long-term results with the "corridor" operation for atrial fibrillation. J Am Coll Cardiol 1991;17:970-5. [PubMed]
- Defauw JJ, Guiraudon GM, van Hemel NM, et al. Surgical therapy of paroxysmal atrial fibrillation with the “corridor” operation. Ann Thorac Surg 1992;53:564-70; discussion 571. [PubMed]
- Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm 2012;9:632-696.e21.
- Cox JL, Canavan TE, Schuessler RB, et al. The surgical treatment of atrial fibrillation. II. Intraoperative electrophysiologic mapping and description of the electrophysiologic basis of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg 1991;101:406-26. [PubMed]
- Cox JL, Schuessler RB, D'Agostino HJ Jr, et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg 1991;101:569-83. [PubMed]
- Cox JL. The surgical treatment of atrial fibrillation. IV. Surgical technique. J Thorac Cardiovasc Surg 1991;101:584-92. [PubMed]
- Cox JL, Schuessler RB, Boineau JP. The surgical treatment of atrial fibrillation. I. Summary of the current concepts of the mechanisms of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg 1991;101:402-5. [PubMed]
- Cox JL, Schuessler RB, Lappas DG, et al. An 8 1/2-year clinical experience with surgery for atrial fibrillation. Ann Surg 1996;224:267-73; discussion 273-5. [PubMed]
- Cox JL, Jaquiss RD, Schuessler RB, et al. Modification of the maze procedure for atrial flutter and atrial fibrillation. II. Surgical technique of the maze III procedure. J Thorac Cardiovasc Surg 1995;110:485-95. [PubMed]
- Nitta T, Ishii Y, Ogasawara H, et al. Initial experience with the radial incision approach for atrial fibrillation. Ann Thorac Surg 1999;68:805-10; discussion 811. [PubMed]
- Cox JL, Ad N, Palazzo T, et al. Current status of the Maze procedure for the treatment of atrial fibrillation. Semin Thorac Cardiovasc Surg 2000;12:15-9. [PubMed]
- Schaff HV, Dearani JA, Daly RC, et al. Cox-Maze procedure for atrial fibrillation: Mayo Clinic experience. Semin Thorac Cardiovasc Surg 2000;12:30-7. [PubMed]
- McCarthy PM, Gillinov AM, Castle L, et al. The Cox-Maze procedure: the Cleveland Clinic experience. Semin Thorac Cardiovasc Surg 2000;12:25-9. [PubMed]
- Prasad SM, Maniar HS, Camillo CJ, et al. The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg 2003;126:1822-8. [PubMed]
- Gaynor SL, Schuessler RB, Bailey MS, et al. Surgical treatment of atrial fibrillation: predictors of late recurrence. J Thorac Cardiovasc Surg 2005;129:104-11. [PubMed]
- Cox JL, Ad N, Palazzo T. Impact of the maze procedure on the stroke rate in patients with atrial fibrillation. J Thorac Cardiovasc Surg 1999;118:833-40. [PubMed]
- Pasic M, Musci M, Siniawski H, et al. Transient sinus node dysfunction after the Cox-maze III procedure in patients with organic heart disease and chronic fixed atrial fibrillation. J Am Coll Cardiol 1998;32:1040-7. [PubMed]
- Sandoval N, Velasco VM, Orjuela H, et al. Concomitant mitral valve or atrial septal defect surgery and the modified Cox-maze procedure. Am J Cardiol 1996;77:591-6. [PubMed]
- Gaynor SL, Diodato MD, Prasad SM, et al. A prospective, single-center clinical trial of a modified Cox maze procedure with bipolar radiofrequency ablation. J Thorac Cardiovasc Surg 2004;128:535-42. [PubMed]
- Damiano RJ Jr, Bailey M. The Cox-Maze IV procedure for lone atrial fibrillation. Multimed Man Cardiothorac Surg 2007;2007:mmcts.2007.002758.
- Weimar T, Bailey MS, Watanabe Y, et al. The Cox-maze IV procedure for lone atrial fibrillation: a single center experience in 100 consecutive patients. J Interv Card Electrophysiol 2011;31:47-54. [PubMed]
- Lall SC, Melby SJ, Voeller RK, et al. The effect of ablation technology on surgical outcomes after the Cox-maze procedure: a propensity analysis. J Thorac Cardiovasc Surg 2007;133:389-96. [PubMed]
- Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659-66. [PubMed]
- de Lima GG, Kalil RA, Leiria TL, et al. Randomized study of surgery for patients with permanent atrial fibrillation as a result of mitral valve disease. Ann Thorac Surg 2004;77:2089-94; discussion 2094-5. [PubMed]
- Bagge L, Blomström P, Nilsson L, et al. Epicardial off-pump pulmonary vein isolation and vagal denervation improve long-term outcome and quality of life in patients with atrial fibrillation. J Thorac Cardiovasc Surg 2009;137:1265-71. [PubMed]
- Han FT, Kasirajan V, Kowalski M, et al. Results of a minimally invasive surgical pulmonary vein isolation and ganglionic plexi ablation for atrial fibrillation: single-center experience with 12-month follow-up. Circ Arrhythm Electrophysiol 2009;2:370-7. [PubMed]
- Edgerton JR, Brinkman WT, Weaver T, et al. Pulmonary vein isolation and autonomic denervation for the management of paroxysmal atrial fibrillation by a minimally invasive surgical approach. J Thorac Cardiovasc Surg 2010;140:823-8. [PubMed]
- Gaita F, Riccardi R, Caponi D, et al. Linear cryoablation of the left atrium versus pulmonary vein cryoisolation in patients with permanent atrial fibrillation and valvular heart disease: correlation of electroanatomic mapping and long-term clinical results. Circulation 2005;111:136-42. [PubMed]
- Tada H, Ito S, Naito S, et al. Long-term results of cryoablation with a new cryoprobe to eliminate chronic atrial fibrillation associated with mitral valve disease. Pacing Clin Electrophysiol 2005;28 Suppl 1:S73-7. [PubMed]
- Boersma LV, Castella M, van Boven W, et al. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST): a 2-center randomized clinical trial. Circulation 2012;125:23-30. [PubMed]
- Barnett SD, Ad N. Surgical ablation as treatment for the elimination of atrial fibrillation: a meta-analysis. J Thorac Cardiovasc Surg 2006;131:1029-35. [PubMed]
- Pison L, La Meir M, van Opstal J, et al. Hybrid thoracoscopic surgical and transvenous catheter ablation of atrial fibrillation. J Am Coll Cardiol 2012;60:54-61. [PubMed]
- Bisleri G, Rosati F, Bontempi L, et al. Hybrid approach for the treatment of long-standing persistent atrial fibrillation: electrophysiological findings and clinical results. Eur J Cardiothorac Surg 2013;44:919-23. [PubMed]
- Muneretto C, Bisleri G, Bontempi L, et al. Successful treatment of lone persistent atrial fibrillation by means of a hybrid thoracoscopic-transcatheter approach. Innovations (Phila) 2012;7:254-8. [PubMed]
- Mahapatra S, LaPar DJ, Kamath S, et al. Initial experience of sequential surgical epicardial-catheter endocardial ablation for persistent and long-standing persistent atrial fibrillation with long-term follow-up. Ann Thorac Surg 2011;91:1890-8. [PubMed]
- Muneretto C, Bisleri G, Bontempi L, et al. Durable staged hybrid ablation with thoracoscopic and percutaneous approach for treatment of long-standing atrial fibrillation: a 30-month assessment with continuous monitoring. J Thorac Cardiovasc Surg 2012;144:1460-5; discussion 1465. [PubMed]
- La Meir M, Gelsomino S, Lorusso R, et al. The hybrid approach for the surgical treatment of lone atrial fibrillation: one-year results employing a monopolar radiofrequency source. J Cardiothorac Surg 2012;7:71. [PubMed]
- Gelsomino S, Van Breugel HN, Pison L, et al. Hybrid thoracoscopic and transvenous catheter ablation of atrial fibrillation. Eur J Cardiothorac Surg 2014;45:401-7. [PubMed]
- Kearney K, Stephenson R, Phan K, et al. A systematic review of surgical ablation versus catheter ablation for atrial fibrillation. Ann Cardiothorac Surg 2014;3:15-29. [PubMed]
- Reddy VY, Doshi SK, Sievert H, et al. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3-Year Follow-up of the PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) Trial. Circulation 2013;127:720-9. [PubMed]
- Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg 1996;61:755-9. [PubMed]
- Bonow RO, Carabello BA, Chatterjee K, et al. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008;118:e523-661. [PubMed]
- Holmes DR, Reddy VY, Turi ZG, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009;374:534-42. [PubMed]
- Healey JS, Crystal E, Lamy A, et al. Left Atrial Appendage Occlusion Study (LAAOS): results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am Heart J 2005;150:288-93. [PubMed]
- García-Fernández MA, Pérez-David E, Quiles J, et al. Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: a transesophageal echocardiographic study. J Am Coll Cardiol 2003;42:1253-8. [PubMed]
- Kanderian AS, Gillinov AM, Pettersson GB, et al. Success of surgical left atrial appendage closure: assessment by transesophageal echocardiography. J Am Coll Cardiol 2008;52:924-9. [PubMed]
- Bakhtiary F, Kleine P, Martens S, et al. Simplified technique for surgical ligation of the left atrial appendage in high-risk patients. J Thorac Cardiovasc Surg 2008;135:430-1. [PubMed]
- Ohtsuka T, Ninomiya M, Nonaka T, et al. Thoracoscopic stand-alone left atrial appendectomy for thromboembolism prevention in nonvalvular atrial fibrillation. J Am Coll Cardiol 2013;62:103-7. [PubMed]
- Slater AD, Tatooles AJ, Coffey A, et al. Prospective clinical study of a novel left atrial appendage occlusion device. Ann Thorac Surg 2012;93:2035-8; discussion 2038-40. [PubMed]
- Bartus K, Han FT, Bednarek J, et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol 2013;62:108-18. [PubMed]
- Massumi A, Chelu MG, Nazeri A, et al. Initial experience with a novel percutaneous left atrial appendage exclusion device in patients with atrial fibrillation, increased stroke risk, and contraindications to anticoagulation. Am J Cardiol 2013;111:869-73. [PubMed]
- Stone D, Byrne T, Pershad A. Early Results With the LARIAT Device for Left Atrial Appendage Exclusion in Patients With Atrial Fibrillation at High Risk for Stroke and Anticoagulation. Catheter Cardiovasc Interv 2013. [Epub ahead of print]. [PubMed]
- Salzberg SP, Plass A, Emmert MY, et al. Left atrial appendage clip occlusion: early clinical results. J Thorac Cardiovasc Surg 2010;139:1269-74. [PubMed]
- Ailawadi G, Gerdisch MW, Harvey RL, et al. Exclusion of the left atrial appendage with a novel device: early results of a multicenter trial. J Thorac Cardiovasc Surg 2011;142:1002-9, 1009.e1.