To avoid compensatory hyperhidrosis after sympathetic surgery for craniofacial hyperhidrosis
Introduction
Hyperhidrosis is a condition of excessive abnormal sweating from stress and/or anxiety, and primary hyperhidrosis is excessive sweating without reason. These conditions can lower the quality of life as it may cause insecurities in social settings and relationships, leading to the potential development of mental illness. Among the various types of primary hyperhidrosis, craniofacial hyperhidrosis (CFH) accounts for approximately 22.8% and is prominent among elderly males (1,2). Given the location of excessive sweating, CFH is easily visible compared with other types of primary hyperhidrosis, and thus, highly associated with social phobia and increased risk of developing psychological issues (2).
To date, endoscopic thoracic sympathectomy (ETS) has been considered the definitive treatment for primary hyperhidrosis (3). It is widely utilized to treat palmar and axillary hyperhidrosis. It has been widely adopted and considered as the definitive treatment, due not only to its relative ease to perform and short recovery time, but more importantly, due to its high success rate (3,4). However, ETS has not been widely adopted in treating CFH because it is known to cause compensatory hyperhidrosis (CH) after its application. The development of post-surgical CH can severely decrease surgical satisfaction (1-3,5). Nonetheless, the exact cause of CH and its treatment remain uncertain.
The cause of primary hyperhidrosis remains unclear; however, the most up-to-date understanding is that it may be due to a failure in the complex function of autonomic nerve system, resulting in neurogenic over-activity of the sweat glands (6). There have been a few studies suggesting that ETS decreases the sympathetic tone and increases the parasympathetic tone, changing the heart rate, and ultimately increasing blood flow in the arteries and lowering blood pressure (7,8). However, to the best of our knowledge, the relationship between CH and autonomic nervous system has not been fully elucidated to date.
Therefore, we sought to determine whether autonomic nerve analysis data, via the pre-ETS heart rate variability (HRV) test, can predict post-ETS CH in patients with CFH.
Methods
Patients
This prospective, observational study included primary CFH patients who consecutively visited the outpatient clinic of the Department of Thoracic and Cardiovascular Surgery at Gangnam Severance Hospital, Seoul, Korea, between October 2017 and March 2019. Patients seeking consultation on the available surgical options to treat primary CFH were included; those with hypertension and associated diseases, such as cardiovascular disease and diabetes mellitus, and those on antidepressants were excluded to avoid influencing the HRV test results. Pre-ETS HRV test was performed on these patients. In brief, all subjects undergoing the pre-ETS HRV test were instructed to have only a light breakfast after an overnight fasting period; patients were also asked to not consume alcohol and caffeine, and to avoid strenuous exercise for a 24-hour period prior to the exam. They were taken to a quiet, dimly-lit room, with a temperature ranging from 22–24 °C. The test was performed between 9:00 pm and 12:00 pm to avoid circadian variation of HRV parameters. All participants rested in supine position for at least 15 minutes on a comfortable bed. Data, including preoperative characteristics, disease status, post-operative outcome, etc. were collected. At the 3-month post-operative follow-up, a survey was conducted to get the status of CH (compensatory grade), which was categorized in the following manner: none; mild CH, defined as sweating occurring in low quantities, triggered by ambient heat, psychological stress, or physical exercise without flow, hence tolerable sweating with minimal embarrassment assessment; moderate CH, defined as sweating occurring in moderate quantity, triggered by ambient heat, psychological stress, or physical exercise that coalesces into droplets with some flow, but not necessitating a change of clothes, thus leading to uncomfortable but tolerable condition; and severe CH, defined as sweating occurring in large quantities, triggered with low or without ambient heat, psychological stress, or physical exercise that coalesces into droplets with heavy flow, requiring a change of clothes several times per day, thus leading to uncomfortable and embarrassing condition. This study was approved by the Institutional Review Board of Gangnam Severance Hospital (3-2019-0181).
ETS technique
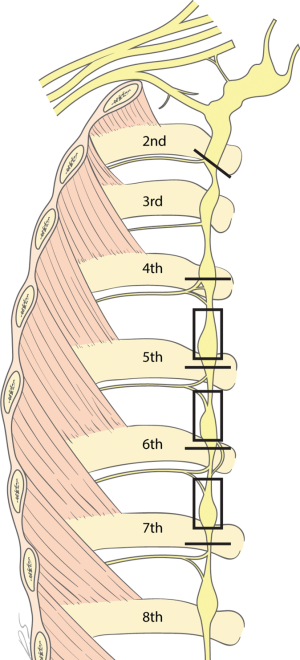
Under general anesthesia, all patients underwent ETS using a bilateral simultaneous two-portal video-assisted thoracoscopic surgery (VATS). The R2 and R4–R7 sympathectomy with R4–R7 truncal ablation using CO2 gas instillation was performed. Two 5-mm incisions were made on each side; one for the thoracoscope at the 6th intercostal space (ICS) on the mid-axillary line, and another for instruments at the 5th ICS on the anterior axillary line. For R2 sympathectomy, the costal pleura on the surface of the sympathetic chain were transected at the second rib lower margin with diathermy. The transection range was extended by about 2 cm, laterally along the surface, to complete a transection of the potential bypass nerve fibers. R4, R5, R6, and R7 sympathicotomies and R4–R7 truncal ablation were performed to prevent upper body CH (Figure 1). The operation was conducted on the right side first to get a view of the imbalanced innervation of the heart by bilateral sympathetic nerves. Following the right side, the same procedure was conducted on the left side. To evacuate inserted CO2 gas, a 10-Fr chest tube was placed in the pleural cavity and the lung was inflated; once the air ceased to escape, the chest tube was removed. Post-operative routine chest radiography was obtained to detect pneumothorax in all patients prior to extubation in the operating room. Most patients were discharged on the same day or on the following day (1).
HRV test
Electrocardiographic data were digitized via an analog-to-digital conversion board (PC-ECG 1200, Norav Medical Ltd., Israel). To verify the beat classification, all records were visually examined and manually read. Any abnormal beats and areas of artifact were identified and excluded, both automatically and manually. The HRV analysis was performed using the HRV Software (version 4.2.0, Norav Medical Ltd., Israel). Time and frequency were analyzed. With respect to time, the mean RR interval (mean RR), standard deviation (SD) of RR interval (SDNN), and the root mean square of successive RR interval differences (RMSSD) were measured. As for frequency, the power spectral analysis, based on the Fast Fourier transformation algorithm, was used. The power spectrum was classified into two categories: high frequency (HF; 0.15–0.4 Hz) and low frequency (LF; 0.04–0.15 Hz). The low frequency/high frequency (LF/HF) value, LF norm, and HF norm were calculated.
Statistical analysis
The demographic and clinical data are presented as the mean ± (SD or frequency (percentage), as appropriate. The baseline characteristics of patients were compared by independent two sample t-test and chi-square test. The area under the curve (AUC) with receiver operating characteristics (ROC) curves was used to measure the predictive ability of the LF/HF value for an event. The maximal chi-square statistics was used to determine the optimal cut-off point. P values of less than 0.05 were considered significant. All statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patients’ characteristics and CH
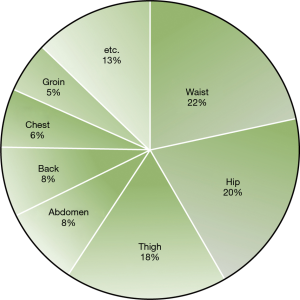
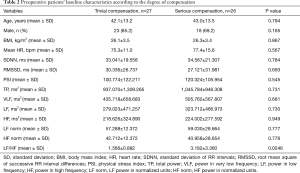
The present study included a total of 53 patients; the average age was 42.5±13.2 years, and there were 41 males (77.4%). The average body mass index (BMI) was 26.2±3.4 kg/m2. Recurrence or failure was not observed in any patient. At 3 months postoperatively, we observed that CH was the most common around the waist area, with 22% of patients showing CH in this area (Figure 2). The compensatory grade distribution was as follows: none in 10 patients (18.9%); mild in 17 patients (32.1%); moderate in 17 patients (32.1%); and severe in 9 patients (17.0%) (Table 1). Based on these grades, patients were divided into (I) the trivial compensation group, which included those with compensatory grades of none and mild, and (II) the serious compensation group, which included those with a grade of moderate and severe. Age, gender, and BMI were not different between these two groups (Table 2).

Full table
Full table
Preoperative HRV test between two groups
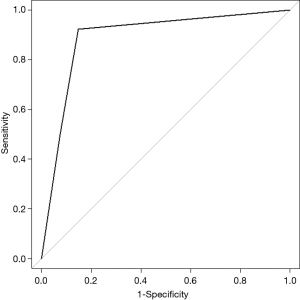
Regarding the HRV test, the mean heart rate, SDNN, RMSSD, physical stress index (PSI), total power (TP), power in very low frequency (VLF), LF, HF, LF power in normalized units (LF norm), and HF power in normalized units (HF norm) were not different between the trivial compensation group and the serious compensation group. However, there was a significant difference between these two groups with respect to the LF/HF value; 1.566±0.882 in the trivial compensation group and 3.182±3.360 in the serious compensation group (Table 2). The optimal cut-off values for the LF/HF, using maximum chi-squared test, were determined to be 0.66 and 2.60, respectively. Hence, if the LF/HF value was less than 0.66, the patient was regarded as parasympathetic dominant; if greater than 0.66 but less than 2.60, the patient was regarded as balanced; and if greater than 2.60, the patient was considered as sympathetic dominant. In the trivial compensatory group, 23 patients (85.2%) were balanced. Conversely, in the serious compensatory group, 11 patients (42.3%) showed parasympathetic dominance, 2 patient (7.7%) balance, and 13 patients (50.0%) sympathetic dominance (Table 3). The predictive ability of the LF/HF value for CH was evaluated using the ROC curve; the AUC (with 95% CI) was 0.890 (0.799–0.982) (P<0.001). Moreover, the sensitivity and specificity of the preoperative LF/HF value (with 95% CI) were 92.3% (74.9–99.1%) and 85.2% (66.3–96.8%), respectively (Figure 3).

Full table
Discussion
CH is the most common and serious side effect of ETS (9). Despite successful completion of surgery, patients experiencing postoperative CH can develop resentment and regret. The outcomes of ETS for primary hyperhidrosis, i.e., postoperative CH, are virtually irreversible (2,4). Several techniques have been proposed in attempts to allow reversibility, including clipping rather than cutting of the sympathetic nerve (10-12). Moreover, a previous case attempted sympathetic chain reconstruction post-ETS (13-15). These strategies may present the potential for improving post-ETS CH; however, they have shown to provide limited value to date.
Since Drott et al. reported the effective use of first ganglion ablation, the success rate of treating CFH has been increasing when compared with other treatment approaches, such as botox injection (16). However, recently, incidence of postoperative CH has dramatically increased, making it difficult to recommend ETS as a first-line treatment for CFH (12). Due to this trend, many institutions have been limiting the use of ETS for treating CFH. At our institution, we recently incorporated a new technique—R2 and R4–R7 sympathectomy with R4–R7 truncal ablation—to redirect post-ETS CH to the lower body rather than to the upper body in hopes for patients to be able to endure the side effect. Based on our trial, 82% of the study population developed postoperative CH, and 48.7% of them rated a compensatory grade of moderate to severe (1). Avoiding the development of CH seems to be a difficult task, especially from just changing the surgical technique. Hence, this study aimed to determine whether HRV can be used to predict the development of postoperative CH prior to undergoing surgery. We believe that this study may be highly meaningful in that we may be able to preemptively determine which patients should avoid ETS and provide a way to select only those with low likelihood of developing postoperative CH as candidates to undergo ETS.
To date, patient factors, including age, gender, BMI, smoking status, and other comorbidities, have been evaluated as potential predictive factors. However, none of these were determined to be significant in predicting postoperative CH (5,9). Moreover, to date, there have been many studies that assessed the level of ETS (R2, R3, and R4) and multi-level sympathectomy as possible risk factors for postoperative CH (17,18). According to Joo et al., R4 sympathicotomy was superior to R3 sympathicotomy in treating palmar hyperhidrosis with respect to the development of postoperative CH (19). However, for treating CFH, R2 sympathicotomy is considered to be the main procedure; hence, we believe that there are no clinical factors that would significantly impact ETS level-related risks of developing postoperative CH. To the best of our knowledge, there are no factors confirmed to influence the risks of developing CH and its associated mechanisms to date. However, among the various factors that were evaluated via HRV test in the present study, LF/HF value showed relatively high sensitivity (92.3%) and specificity (85.2%), compared with any other factors evaluated to date, in its ability to predict postoperative CH.
We were able to divide CFH patients into the autonomic balanced group and the autonomic dysfunction group, based on the LF/HF value obtained from the preoperative HRV test; patients with an LF/HF value in between 0.66 and 2.60 were placed into the autonomic balanced group, while those with a value of less than 0.66 or greater than 2.60 were put into the autonomic dysfunction group. In the autonomic balanced group, 8% developed postoperative serious CH; conversely, 85.7% in the autonomic dysfunction group developed postoperative serious CH. It has been established that primary hyperhidrosis has various environmental and psychological triggers, and is a result of a hyper-response or over-activity of the sweating center, which is regulated in the neocortex and limbic system levels (7,20). Moreover, it is widely believed that hyperhidrosis may be due to a failure of the complex function of the autonomic nerve system, inducing the neurogenic over-activity of the sweat glands; and as such, there have been many pathophysiologic studies (6). However, to the best of our knowledge, the autonomic nerve system with respect to CH and its related mechanism have not been fully elucidated. The present study showed that there was less incidence of post-ETS CH in the autonomic balanced group. We believe that the risk of developing post-ETS CH may be lowered if the parasympathetic capacity is reinforced and supplemented via sympathetic nerve transection. Therefore, there may be increased risk of developing post-ETS CH in CFH patients accompanied with autonomic dysfunction.
There are several limitations to this study. First, the number of patients included in this study was small, and it was a single-center study. Second, the compensatory grade for CH was measured subjectively. To our knowledge, there currently is no method of quantifying the degree or severity of CH. Third, given the characteristics of the HRV test and variations in the results presented, there may be a small measurement error. However, to minimize variation, we attempted to exclude factors that may influence the results of the HRV test and to perform the HRV test under the same condition. Nonetheless, a future study with greater study population with long-term follow-up may be necessary.
In conclusion, our findings suggest that HRV testing on CFH patients may be useful in predicting the likelihood of developing post-ETS CH. In the future, studies evaluating the pathophysiology and mechanism of CH-related autonomic nerve system may be necessary.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.28). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All patient information was collected in accordance with the tenets of the Declaration of Helsinki and the Health Insurance Portability Act. The study was approved by the institutional review board at the Gangnam Severance Hospital (3-2019-0181).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Moon DH, Kang DY, Kim DW, et al. Early results of new endoscopic thoracic sympathectomy for craniofacial hyperhidrosis. J Thorac Dis 2018;10:3627-31. [Crossref] [PubMed]
- Nicholas R, Quddus A, Baker DM. Treatment of Primary Craniofacial Hyperhidrosis: A Systematic Review. Am J Clin Dermatol 2015;16:361-70. [Crossref] [PubMed]
- Cerfolio RJ, De Campos JR, Bryant AS, et al. The Society of Thoracic Surgeons expert consensus for the surgical treatment of hyperhidrosis. Ann Thorac Surg 2011;91:1642-8. [Crossref] [PubMed]
- Guerra M, Neves PC. Thoracoscopic sympathectomy for hyperhidrosis. Rev Port Cir Cardiotorac Vasc 2011;18:77-83. [PubMed]
- Cameron AE. Selecting the Right Patient for Surgical Treatment of Hyperhidrosis. Thorac Surg Clin 2016;26:403-6. [Crossref] [PubMed]
- Chou SH, Kao EL, Lin CC, et al. The importance of classification in sympathetic surgery and a proposed mechanism for compensatory hyperhidrosis: experience with 464 cases. Surg Endosc 2006;20:1749-53. [Crossref] [PubMed]
- Fiorelli A, Messina G, Chiodini P, et al. Cardiac Autonomic Changes After Thoracic Sympathectomy: A Prospective, Randomized Study. Ann Thorac Surg 2017;103:216-24. [Crossref] [PubMed]
- Liu G, Kang G, Huang J, et al. Changes in Palm Temperature as Predictor of Long-term Cure of Sympathicotomy for Palmar hyperhidrosis? J Neurol Surg A Cent Eur Neurosurg 2019;80:67-71. [Crossref] [PubMed]
- Ruan GJ, Thuppal S, Sawyer JD, et al. Compensatory Hyperhidrosis and Quality of Life Post Sympathectomy for Palmar Hyperhidrosis. Am Surg 2019;85:438-40. [PubMed]
- Fibla Alfara JJ, Molins Lopez-Rodo L, Hernandez Ferrandez J, et al. Effectiveness of bilateral clipping of the thoracic sympathetic chain for the treatment of severe palmar and/or axillary hyperhidrosis and facial flushing. Cir Esp 2019;97:196-202. [Crossref] [PubMed]
- Onugha O, Ivey R, McKenna R. Novel Techniques and Approaches to Minimally Invasive Thoracic Surgery. Surg Technol Int 2017;30:231-5. [PubMed]
- Wolosker N, Milanez de Campos JR, Fukuda JM. Management of Compensatory Sweating After Sympathetic Surgery. Thorac Surg Clin 2016;26:445-51. [Crossref] [PubMed]
- Jung HS, Lee DY, Park JS. Alternative Surgical Methods in Patients with Recurrent Palmar Hyperhidrosis and Compensatory Hyperhidrosis. Yonsei Med J 2018;59:345-48. [Crossref] [PubMed]
- Park HS, Hensman C, Leong J. Thoracic sympathetic nerve reconstruction for compensatory hyperhidrosis: the Melbourne technique. Ann Transl Med 2014;2:45. [PubMed]
- Rantanen T, Telaranta T. Long-Term Effect of Endoscopic Sympathetic Nerve Reconstruction for Side Effects after Endoscopic Sympathectomy. Thorac Cardiovasc Surg 2017;65:484-90. [Crossref] [PubMed]
- Drott C. Results of endoscopic thoracic sympathectomy (ETS) on hyperhidrosis, facial blushing, angina pectoris, vascular disorders and pain syndromes of the hand and arm. Clin Auton Res 2003;13 Suppl 1:I26-30. [Crossref] [PubMed]
- Sternbach JM, DeCamp MM. Targeting the Sympathetic Chain for Primary Hyperhidrosis: An Evidence-Based Review. Thorac Surg Clin 2016;26:407-20. [Crossref] [PubMed]
- Zhang W, Yu D, Wei Y, et al. A systematic review and meta-analysis of T2, T3 or T4, to evaluate the best denervation level for palmar hyperhidrosis. Sci Rep 2017;7:129. [Crossref] [PubMed]
- Joo S, Lee GD, Haam S, et al. Comparisons of the clinical outcomes of thoracoscopic sympathetic surgery for palmar hyperhidrosis: R4 sympathicotomy versus R4 sympathetic clipping versus R3 sympathetic clipping. J Thorac Dis 2016;8:934-41. [Crossref] [PubMed]
- Okutucu S, Aksoy H, Oto A. Complex autonomic pathways in patients with idiopathic hyperhidrosis. Clin Exp Dermatol 2017;42:797-8. [Crossref] [PubMed]