
Predictors of survival following veno-arterial extracorporeal membrane oxygenation in patients with acute myocardial infarction-related refractory cardiogenic shock: clinical and coronary angiographic factors
Introduction
Cardiogenic shock is a life-threatening emergency with a high mortality rate (1). In patients with acute myocardial infarction (AMI), about 7–10% are complicated by cardiogenic shock, which is associated with a high mortality rate of 50–70% (2). Increasingly, severe cases of refractory cardiogenic shock (RCS) are managed with mechanical circulatory support such as veno-arterial extracorporeal membrane oxygenation (ECMO), which can be a bridge-to-recovery of cardiac function (3). Reports indicate that ECMO could be effectively administered to patients with AMI-related RCS and suggest the survival advantage of early ECMO-assisted primary percutaneous coronary intervention (PCI) in such circumstances (4).
In patients with AMI, favorable outcomes were reported after primary PCI. However, worse reperfusion rates and unfavorable remodeling are often observed in patients with multivessel coronary artery disease (MVCAD), which suggests that the extent of non-culprit coronary disease may directly or indirectly impact the clinical outcome (5,6). Even after successful PCI, patients with AMI due to left main coronary artery disease (LMCAD) showed poor clinical outcomes and high mortality rates due to combined RCS (7,8). To date, only a few studies have specifically focused on clinical factors in ECMO-treated AMI patients with RCS, and none has considered the coronary angiographic findings and the clinical prognostic scoring system together (9,10). Therefore, we investigated whether the simplified acute physiology score II (SAPSII), sepsis-related organ failure assessment score (SOFA), and survival after veno-arterial extracorporeal membrane oxygenation score (SAVE), as well as coronary angiographic findings could be predictors of survival in patients with AMI-related RCS treated with ECMO.
Methods
Study population
Patients were eligible for enrollment if they were >30 and <85 years of age. AMI patients who underwent primary PCI and received ECMO because of AMI-related RCS were included. Patients were excluded if they were ≥85 years of age or if they had any of the following conditions: a history of significant valvular heart disease (more than severe degree), unwitnessed cardiac arrest, ongoing intracranial hemorrhage, chronic severe organ dysfunction (such as emphysema and liver cirrhosis), severe immunosuppression, end-stage malignancy, or prolonged cardiopulmonary resuscitation (CPR) without adequate tissue perfusion. In this study, RCS was defined as cardiac and circulatory failure resulting in a systolic pressure of <90 mmHg and organ hypoperfusion unresponsive to conventional medical therapies including vasoactive medications (11). This study was approved by the institutional review board of Hallym University Sacred Heart Hospital.
CPR, ECMO, and coronary angiographic related factors
Including laboratory findings, SAPSII, SOFA, and SAVE, CPR-related characteristics such as cardiac arrest, out of hospital cardiac arrest (OHCA), ventricular tachycardia or ventricular fibrillation were evaluated. Total CPR time was calculated by subtracting the return of spontaneous circulation time from the total chest compression time. ECMO-related factors such as extracorporeal membrane oxygenation cardiopulmonary resuscitation (ECPR), collapse to ECMO initiation time, continuous renal replacement therapy, intra-aortic balloon pump (IABP), ECMO duration, length of intensive care unit stay and length of hospital stay were evaluated. Coronary angiographic and PCI-related findings such as door-to-balloon time, culprit lesion of coronary artery, LMCAD, chronic total occlusion, and number of coronary artery disease (NCAD) were evaluated. In this study, the SAPSII, SOFA, and SAVE scores were modified to collect the relevant pre-ECMO data from patients just prior to deciding ECMO. Significant coronary artery disease was defined as a lumen diameter stenosis ≥70% in the major coronary arteries (diameter ≥2.5 mm) as determined by coronary angiography (CAG). The LMCAD was counted as a two-vessel disease. CAG was interpreted by an interventional cardiologist (WJ Park) blinded to the patients’ clinical data.
Initiation and management of ECMO
Most of the patients were managed with the Permanent Life Support System (MAQUET, Rastatt, Germany), while two patients were treated with the Capiox Emergency Bypass System (Terumo, Tokyo, Japan). Depending on the patient, 17–21 Fr arterial cannula and 17–23 Fr venous cannula (HLS cannula, MAQUET, Rastatt, Germany or Bio-Medicus, Medtronic, Minneapolis, MN, USA) were used. Just before ECMO cannulation, all patients received 3,000–5,000 IU of intravenous unfractionated heparin. VA-ECMO was cannulated percutaneously using the Seldinger technique under fluoroscopic guidance in a cardiac catheterization laboratory or hybrid operating room in the emergency department. The initial gas and blood flow rates were 4–6 and 3–5 L/min, respectively, to maintain oxygen saturation (SpO2) >90%. During ECMO support, heparin or nafamostat mesilate (SK Chemicals Life Science Biz., Seoul, Korea) was used for anticoagulation, with a target activated partial thromboplastin time of 60–80 seconds. After the initiation of ECMO, we tried to maintain a cardiac index of ≥2.0 L/min/m2 by adjusting ECMO flow, a mean arterial blood pressure of 70–75 mmHg, and a mixed venous oxygen saturation level of around 70%. All patients were sedated with morphine, midazolam, remifentanil, dexmedetomidine, or fentanyl, and the body temperature was maintained between 36–37 °C with a membrane oxygenator heat exchanger. Blood transfusions comprising packed red blood cells with hemoglobin concentration of <8–10 g/dL, fresh frozen plasma with an international normalized ratio of >2.0, platelet concentrate with a platelet count of <50,000/µL and cryoprecipitate with a fibrinogen concentration of <150 mg/dL were administered.
Weaning from ECMO
The criteria for weaning included stable vital signs, no definite bleeding foci, mixed venous oxygen saturation of ≥70%, absence of tamponade or left heart distension, left ventricular ejection fraction ≥35%, and normalized lactic acid level in blood. The ECMO flow rate was reduced stepwise under continuous monitoring of hemodynamic and respiratory variables. In this study, successful ECMO weaning was defined as weaning followed by stable survival for more than 48 hours. Survival after ECMO was defined as successful weaning and treatment of the underlying medical condition, followed by 100-day survival without any further adverse events.
Statistical analysis
The continuous variables were summarized as mean ± SD. The categorical variables were presented as numbers or percentages. The continuous and categorical variables were analyzed by the independent samples t-test or the Mann-Whitney U-test, and the Pearson chi-square test or the Fisher’s exact test, as appropriate. The univariate and multivariate stepwise logistic regression analysis models were used to identify independent non-survivor-related factors. After univariate logistic regression analysis, variables with a level of statistical significance of P<0.050 were entered as potential candidate variables in the multivariate logistic regression models to assess the independent predictors for mortality in this study. To evaluate and compare the predictive power of the non-survivor factors, a receiver operating characteristic (ROC) analysis was performed, and the area under the curve (AUC) was calculated for SAPSII, SOFA, and SAVE. Compared with SAPSII and SOFA, the greater negative number values of SAVE predicts a higher mortality rate. The maximum negative value of the SAVE score is −35 points. Therefore, to compare the SAPSII, SOFA, and SAVE scores using ROC analysis, the value of 35 minus the original SAVE score was used to calculate the positive value. In this study, NCAD was evaluated as a combined parameter of clinical prediction scores of mortalities. To compare the combined parameters SAPSII plus NCAD, SOFA plus NCAD, and SAVE plus NCAD, one, two, and three points were added for one-vessel disease, two-vessel disease, and three-vessel disease, respectively. The value of the SAPSII score is nearly 10 times that of the SOFA score as well as of the positive value of the SAVE score; the NCAD point multiplied by 10 was added to SAPSII score. Data were analyzed using standard statistical software (SPSS version 19.0; SPSS Inc., Chicago, IL, USA and MedCalc version 13.0; MedCalc Software, Mariakerke, Belgium). A probability value of P<0.050 was considered statistically significant.
Results
Between January 2015 and July 2019, 71 consecutive AMI-induced RCS patients who received ECMO and PCI at the Hallym University Sacred Heart hospital were evaluated. Of the 71 patients, two were excluded (one patient had severe aortic stenosis and the other was 87 years old). The survivor group consisted of 30 (43.4%) patients who survived for more than 100 days. The causes of death of the non-survivor group included multi-organ failure (n=13), sepsis or systemic inflammatory response syndrome (n=9), hypoxic brain damage (n=8), pneumonia (n=4), heart failure (n=2), aortic dissection (n=1), myocardial infarction-related ventricular septal defect (n=1), and pulmonary hemorrhage (n=1).
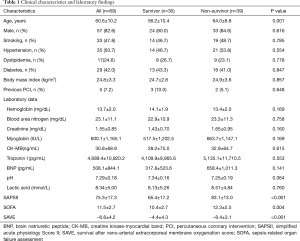
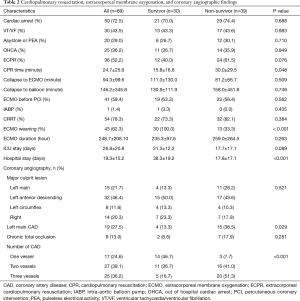
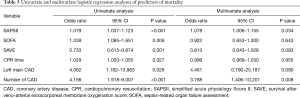
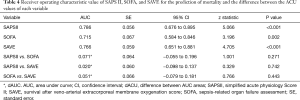
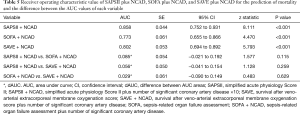
The baseline clinical characteristics and laboratory findings of the survivor group (n=30) and the non-survivor group (n=39) are shown in Table 1. The SAPSII and SOFA scores were significantly higher in positive value and the SAVE scores significantly lower in negative value in the non-survivor group. Table 2 shows the CPR, ECMO, and coronary angiographic findings of the study. The CPR time was significantly longer in the non-survivor group than the survivor group. The coronary angiographic findings showed that the major culprit lesion was the left anterior descending artery but there was no difference in the culprit lesion between the groups. The incidence of LMCAD was significantly higher in the non-survivor group than in the survivor group and the NCAD was significantly different between the groups. Table 3 shows the univariate and multivariate analysis of the predictors of mortality. The univariate analysis showed that SAPSII, SOFA, SAVE, CPR time, LMCAD, and NCAD were significantly associated with mortality-related factors in the study. However, the multivariate analysis revealed that only SAPSII and NCAD were independent predictors of mortality in the study (OR 1.078, 95% CI: 1.006–1.155, P=0.034 and OR 3.788, 95% CI: 1.406–10.201, P=0.008, respectively). The ROC analysis showed that SAPSII, SOFA, and SAVE all significantly predicts mortality and there was no difference among the three scores (Table 4). Table 5 shows that the ROC values of SAPSII plus NCAD, SOFA plus NCAD, and SAVE plus NCAD all significantly predicts mortality and there was no difference among the three scores. However, there was significant difference in the AUC values (dAUC) between SAPSII plus NCAD vs. SAPSII, SOFA plus NCAD vs. SOFA, and SAVE plus NCAD vs. SAVE in predicting mortality in the study (Table 6).
Full table
Full table
Full table
Full table
Full table

Full table
Discussion
This study demonstrates that the SAPSII, SOFA, and SAVE scores can predict the prognosis in patients treated with ECMO due to AMI-related RCS and the coronary angiographic findings can enhance the predictive power of the score-based prognosis. The current study demonstrates for the first time that the clinical scoring system and angiographic severity of the coronary artery disease are essential for predict the prognosis in patients with ECMO-treated AMI-related RCS.
ECMO is a modified form of cardiopulmonary bypass and has undergone a dramatic evolution since early 1970s (12). Technical improvements have contributed to the current worldwide use of ECMO, which has been a valid treatment device for RCS unresponsive to medical therapy (1). In AMI patients with RCS, IABP has been used. However, the IABP-SHOCK II trial reported that IABP did not reduce the 30-day mortality in patients with RCS-complicating AMI (13). Therefore, the main option available for AMI-related RCS would be a mechanical circulatory support device such as ECMO. Although there is broad use of ECMO in experienced treatment centers, studies reporting short-term and long-term prognostic factors of ECMO-treated RCS are limited (4,14). A few studies have focused on the clinical results of ECMO in patients with AMI-related RCS, which is the main cause of early mortality in patients with AMI (9,10).
Since the introduction of early revascularization in AMI, mortality due to AMI-induced RCS has markedly decreased over the last three decades, but recent trials have reported that the mortality still remains high, at 45–60% (2,15). In patients with AMI-complicated cardiac arrest or RCS, CPR is a poor independent predictor of in-hospital mortality (16). Most studies of conventional CPR showed that the duration of CPR is independently associated with poor functional outcomes and survival rates (17,18). Therefore, ECMO should be promptly considered in patients with AMI complicated with cardiac arrest or RCS in order to reduce the CPR duration and avoid CPR-related complications that may impair the survival rate (9). In this study, the total CPR time was significantly shorter in the survivor group than in the non-survivor group, and the univariate analysis showed that the CPR time significantly predicted mortality in the study. Therefore, in patients with RCS induced by AMI, the most helpful treatment appears to be the prompt application of ECMO to avoid prolonged CPR, reducing the amounts of inotropic agents, and opening the occluded coronary artery in stable condition. When a patient with AMI-complicated RCS arrives at the emergency department, the timely use of ECMO should always be considered.
In patients with AMI-related RCS, it is essential to determine the appropriate candidate for ECMO, but this may vary based on the physicians and the treatment centers. Therefore, identification and evaluation of pre-ECMO predictors is indispensable in predicting survival of the patients. There is no universally accepted ECMO-specific risk scoring system to predict early and late prognosis. The standard risk scores such as SAPSII and SOFA have been used to help assess mortality of patients in intensive care units (19,20). Studies have reported that the SAPSII and SOFA scores might be useful for predicting survival and successful weaning in patients undergoing ECMO in emergency departments or intensive care units (21,22). Schmidt et al. reported that the SAVE score is a potential tool to predict in-hospital survival for patients receiving ECMO due to RCS (14). In this study, there were significant differences in the SAPSII, SOFA, and SAVE scores between the survivor and non-survivor groups. The ROC analysis showed that the SAPSII, SOFA, and SAVE scores significantly predicted mortality and that there was no difference in the AUC values among the scores. This means that the SAPSII, SOFA and SAVE scoring system could predict mortality in ECMO-treated AMI patients with RCS.
Although mortality due to AMI-related RCS has markedly decreased due to early revascularization, it remains unacceptably high (2,23). Regarding the anatomical aspects of coronary artery lesions, studies have reported that LMCAD and NCAD were independently associated with in-hospital death in patients with AMI-related RCS (24,25). AMI due to LMCAD is catastrophic. Although not all patients with left main AMI are able to undergo emergency CAG for confirmation, 1–2% of the AMI patients have LMCAD as a culprit lesion (26). In these patients, the incidence of cardiogenic shock is as high as 50–80%, and even after receiving successful reperfusion therapy, their acute-phase mortality may be as high as 40–60% (7,8). In our study, 4 patients among the non-survivors had LMCAD as a combined non-culprit lesion. A total of 19 (27.5%) patients had LMCAD and the incidence of LMCAD was significantly higher in the non-survivor group than in the survivor group.
The main goal of PCI in the setting of AMI is to re-perfuse the myocardium by opening the occluded culprit coronary artery. However, in addition to infarct-related culprit lesion, 50–80% of the AMI patients have MVCAD, which is highly associated with adverse clinical outcomes (5,27). Studies have reported that compared with single vessel coronary artery disease, a higher incidence of type C complex lesions and a lower incidence of myocardial blush grade 3 were observed among the AMI patients with MVCAD (6,28). The increased risk in AMI patients with MVCAD could be explained by the effects of the remaining extensive coronary atherosclerosis, such as the presence of stunned and hibernating myocardium, impaired ventricular function in the non-infarct area, and a slow flow in critically narrowed non-infarct related arteries that could offset the effect of circulatory support of ECMO (5,29). In this study, 52 (75.4%) patients had MVCAD and the MVCAD rates were higher in the non-survivor group than in the survivor group [36 (92.3%) vs. 16 (53.3%), P<0.001]. In this study, the multivariate analysis showed that NCAD is an independent predictor of mortality. From the ROC analysis, the combined parameters of NCAD and clinical risk scores showed that the SAPSII plus NCAD, SOFA plus NCAD, and SAVE plus NCAD scores have significantly better predictability for mortality compared with the single-parameter SAPSII, SOFA, and SAVE scores. Therefore, even after the successful PCI of the infarct-related artery, the presence of significant concomitant coronary artery disease which is remote from the culprit lesion should be recognized as an important prognostic parameter in patients treated with ECMO due to AMI-related RCS.
Limitations
Several limitations of our study should be considered. First, this study was conducted at a single institution and the study population was relatively small, though all the patients had AMI-related RCS, the specific underlying disease for which veno-arterial ECMO is indicated. Second, the processes of initiation and management of ECMO may differ based on the treatment centers which may influence the outcomes. Third, this study was performed with 100-day survival as a clinical result. Therefore, additional large-scale multicenter studies with long-term outcomes will be needed to confirm and generalize our results.
Conclusions
SAPSII, SOFA, and SAVE scores could predict survival in patients treated with ECMO due to AMI-related RCS. The coronary angiographic findings enhance the prognostic predictive power of the scores. Therefore, both the clinical scoring system and the angiographic severity of the coronary artery disease are essential to predict the prognosis in patients with ECMO-treated AMI-related RCS.
Acknowledgments
Funding: The research was supported by the National Research Foundation of Korea funded by the Ministry of Science, ICT, & Future Planning (NRF-2017R1D1A3B03028830) to KHP.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.51). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the institutional review board of Hallym University Sacred Heart Hospital.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Napp LC, Kuhn C, Bauersachs J. ECMO in cardiac arrest and cardiogenic shock. Herz 2017;42:27-44. [Crossref] [PubMed]
- Babaev A, Frederick PD, Pasta DJ, et al. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. Jama 2005;294:448-54. [Crossref] [PubMed]
- Stretch R, Sauer CM, Yuh DD, et al. National trends in the utilization of short-term mechanical circulatory support: incidence, outcomes, and cost analysis. J Am Coll Cardiol 2014;64:1407-15. [Crossref] [PubMed]
- Sheu JJ, Tsai TH, Lee FY, et al. Early extracorporeal membrane oxygenator-assisted primary percutaneous coronary intervention improved 30-day clinical outcomes in patients with ST-segment elevation myocardial infarction complicated with profound cardiogenic shock. Crit Care Med 2010;38:1810-7. [Crossref] [PubMed]
- Sorajja P, Gersh BJ, Cox DA, et al. Impact of multivessel disease on reperfusion success and clinical outcomes in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Eur Heart J 2007;28:1709-16. [Crossref] [PubMed]
- de Waha S, Eitel I, Desch S, et al. Impact of multivessel coronary artery disease on reperfusion success in patients with ST-elevation myocardial infarction: A substudy of the AIDA STEMI trial. Eur Heart J Acute Cardiovasc Care 2017;6:592-600. [Crossref] [PubMed]
- Izumikawa T, Sakamoto S, Takeshita S, et al. Outcomes of primary percutaneous coronary intervention for acute myocardial infarction with unprotected left main coronary artery occlusion. Catheter Cardiovasc Interv 2012;79:1111-6. [Crossref] [PubMed]
- Lee SW, Hong MK, Lee CW, et al. Early and late clinical outcomes after primary stenting of the unprotected left main coronary artery stenosis in the setting of acute myocardial infarction. Int J Cardiol 2004;97:73-6. [Crossref] [PubMed]
- Han SJ, Kim HS, Choi HH, et al. Predictors of survival following extracorporeal cardiopulmonary resuscitation in patients with acute myocardial infarction-complicated refractory cardiac arrest in the emergency department: a retrospective study. J Cardiothorac Surg 2015;10:23. [Crossref] [PubMed]
- Muller G, Flecher E, Lebreton G, et al. The ENCOURAGE mortality risk score and analysis of long-term outcomes after VA-ECMO for acute myocardial infarction with cardiogenic shock. Intensive Care Med 2016;42:370-8. [Crossref] [PubMed]
- Beurtheret S, Mordant P, Paoletti X, et al. Emergency circulatory support in refractory cardiogenic shock patients in remote institutions: a pilot study (the cardiac-RESCUE program). Eur Heart J 2013;34:112-20. [Crossref] [PubMed]
- Napp LC, Kuhn C, Hoeper MM, et al. Cannulation strategies for percutaneous extracorporeal membrane oxygenation in adults. Clin Res Cardiol 2016;105:283-96. [Crossref] [PubMed]
- Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287-96. [Crossref] [PubMed]
- Schmidt M, Burrell A, Roberts L, et al. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J 2015;36:2246-56. [Crossref] [PubMed]
- Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization and long-term survival in cardiogenic shock complicating acute myocardial infarction. Jama 2006;295:2511-5. [Crossref] [PubMed]
- Park TK, Yang JH, Choi SH, et al. Clinical outcomes of patients with acute myocardial infarction complicated by severe refractory cardiogenic shock assisted with percutaneous cardiopulmonary support. Yonsei Med J 2014;55:920-7. [Crossref] [PubMed]
- Hajbaghery MA, Mousavi G, Akbari H. Factors influencing survival after in-hospital cardiopulmonary resuscitation. Resuscitation 2005;66:317-21. [Crossref] [PubMed]
- Reynolds JC, Frisch A, Rittenberger JC, et al. Duration of resuscitation efforts and functional outcome after out-of-hospital cardiac arrest: when should we change to novel therapies? Circulation 2013;128:2488-94. [Crossref] [PubMed]
- Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. Jama 1993;270:2957-63. [Crossref] [PubMed]
- Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996;22:707-10. [Crossref] [PubMed]
- Lee HS, Kim HS, Lee SH, et al. Clinical implications of the initial SAPS II in veno-arterial extracorporeal oxygenation. J Thorac Dis 2019;11:68-83. [Crossref] [PubMed]
- Chang WW, Tsai FC, Tsai TY, et al. Predictors of mortality in patients successfully weaned from extracorporeal membrane oxygenation. PLoS One 2012;7:e42687. [Crossref] [PubMed]
- Klein LW, Shaw RE, Krone RJ, et al. Mortality after emergent percutaneous coronary intervention in cardiogenic shock secondary to acute myocardial infarction and usefulness of a mortality prediction model. Am J Cardiol 2005;96:35-41. [Crossref] [PubMed]
- Zeymer U, Vogt A, Zahn R, et al. Predictors of in-hospital mortality in 1333 patients with acute myocardial infarction complicated by cardiogenic shock treated with primary percutaneous coronary intervention (PCI); Results of the primary PCI registry of the Arbeitsgemeinschaft Leitende Kardiologische Krankenhausarzte (ALKK). Eur Heart J 2004;25:322-8. [Crossref] [PubMed]
- Sanborn TA, Sleeper LA, Webb JG, et al. Correlates of one-year survival in patients with cardiogenic shock complicating acute myocardial infarction: angiographic findings from the SHOCK trial. J Am Coll Cardiol 2003;42:1373-9. [Crossref] [PubMed]
- Parma A, Fiorilli R. Early and mid-term clinical outcome of emergency PCI in patients with STEMI due to unprotected left main coronary artery disease. J Interv Cardiol 2012;25:215-22. [Crossref] [PubMed]
- Corpus RA, House JA, Marso SP, et al. Multivessel percutaneous coronary intervention in patients with multivessel disease and acute myocardial infarction. Am Heart J 2004;148:493-500. [Crossref] [PubMed]
- van der Schaaf RJ, Timmer JR, Ottervanger JP, et al. Long-term impact of multivessel disease on cause-specific mortality after ST elevation myocardial infarction treated with reperfusion therapy. Heart 2006;92:1760-3. [Crossref] [PubMed]
- Tarantini G, Napodano M, Gasparetto N, et al. Impact of multivessel coronary artery disease on early ischemic injury, late clinical outcome, and remodeling in patients with acute myocardial infarction treated by primary coronary angioplasty. Coron Artery Dis 2010;21:78-86. [Crossref] [PubMed]


