
Multimodal approach to the management of malignant pleural effusions: role of thoracoscopy with pleurodesis and tunneled indwelling pleural catheters
Introduction
Malignant pleural effusion (MPE) is associated with a median survival of 3–6 months (74 days in patients with lung cancer) and affects the overall quality of life (1-3). Approximately 150,000 cases of MPE’s occur annually in the United States and more than 50% of the patients re-accumulate fluid after initial drainage (4,5). One recent study showed that 30% of the patients with MPE had fluid recurrence at day 15 post thoracentesis, 40% at day 30 and 48% had recurrence at day 90. In a larger SEER-Medicare data base analysis, the overall recurrence of MPEs was noted in 55% (12,967/23,431) patients with 58% of those with recurrence having a rapid re-accumulation (within 2 weeks). Despite the high and relatively rapid recurrence rates, only 23% of the patients had a definitive pleural procedure with the rest undergoing repeat thoracentesis. Even after further episodes of recurrence, a similar percentage of patients underwent a definitive pleural procedure with overall guideline consistent care being followed in only 24% of the patients. Definitive pleural procedures [interventions performed to prevent recurrent presentation with dyspnea and minimize symptoms/repeated procedures such as pleurodesis or tunneled indwelling pleural catheter placement (TIPC)] compared with repeat thoracentesis resulted in fewer subsequent pleural procedures, fewer complications (e.g., pneumothoraces) and fewer procedures in the emergency department (5). In addition, it is known that repeated pleural procedures are associated with decreased quality of life preceding the procedure (6).
It is thus relevant that guidelines-consistent care be provided in patients with MPE. In this review, we summarize the guidelines for management of MPE by various societies and discuss a multimodal approach in this patient population by using thoracoscopy with talc insufflation pleurodesis and placement of TIPC.
Summary of the current guidelines on MPE
The American Thoracic Society (ATS) published its initial guidelines for the management of patients with MPE in 2000. The British Thoracic Society published its own guidelines in 2010. Over the last decade, however, several large trials have been completed and there has been significant progress in the evidence-based management of patients with MPE. Thus the ATS, The Society of Thoracic Surgeons (STS) and the Society of Thoracic Radiology (STR) developed new evidence-based recommendations for the management of these patients (7). Similarly the European Respiratory Society (ERS) and the European Association for Cardio-Thoracic Surgery (EACTS) also published updated guidelines for the management if MPE in 2018 (8). The European Society of Medical Oncology (ESMO) and National Comprehensive Cancer Network (NCCN) also provide recommendations related to patients with lung cancer who develop MPE (9). The recommendations from these societies are summarized in Table 1.

Full table
Diagnostic thoracoscopy for pleural biopsy
Thoracentesis is the first step in the evaluation of pleural effusion and a single pleural aspiration is diagnostic of malignancy in about 40–60% of cases (10,11). The diagnostic yield varies based upon the type of the solid tumor with the sensitivity of pleural fluid cytology ranging from 38% in head and neck cancers to 93% and 100% in patients with breast cancer and pancreatic cancer, respectively (11). In patients with an undiagnosed exudative effusion with a high suspicion of malignancy, a thoracoscopic approach with pleural biopsy should be performed to provide a firm diagnosis. The diagnostic sensitivity of thoracoscopy in this subset of patients is 90% to 100% (12-14). The use of autofluorescence mode and narrow-band imaging has been studied but has not shown any significant advantages compared to white light thoracoscopy in obtaining pleural biopsies (15,16). Pleural tissue sampling can be accomplished using the standard rigid forceps or the flexible forceps passed through the working channel of a semi-rigid thoracoscope. A recent systematic review assessed the diagnostic yield of pleural cryobiopsy compared to flexible forceps biopsy and showed a comparable safety profile but did not demonstrate an increase in the diagnostic yield (17). In patients with recurrent MPE, talc insufflation and/or TIPC can be performed in the same setting during the diagnostic thoracoscopy.
Thoracoscopy with talc insufflation/poudrage
Given the high recurrence rates of MPE, chemical pleurodesis plays an integral role in management of these patients, especially in patients with an expandable lung. Chemical pleurodesis can be performed by instilling a sclerosant via the chest tube or via talc insufflation/poudrage during a thoracoscopy. Thoracoscopic talc insufflation is done after fluid aspiration and pleural biopsy using a spray atomizer via the trocar (Figure 1) or by using a catheter through the working channel of the semi-rigid pleuroscope.
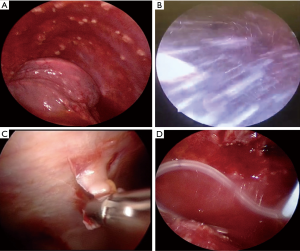
The use of thoracoscopic talc insufflation for MPE has been reported in multiple studies (8,18-27). While the dose of talc and the definition of pleurodesis across these reports have been variable, the success rate has ranged from 77% to 98% with complication rates ranging from 2% to 17.2%. Despite the initial concern for ARDS with talc pleurodesis, no ARDS was noted in 558 patients post pleurodesis with graded talc (26).
Herein we provide a narrative review of landmark original studies and meta-analyses pertinent to talc pleurodesis. Yim et al. (28) conducted a randomized trial comparing video-assisted thoracoscopic talc insufflation with bedside talc slurry in 57 patients with an expandable lung. No difference was shown between the 2 groups in terms of duration of chest tube, length of stay, complications or recurrence of pleural effusion. Similar findings were reported by Mummadi et al. in their systematic review and meta-analysis with no overall difference in pleurodesis noted between thoracoscopic talc insufflation and talc slurry via a chest tube (RR 1.06, 95% CI, 0.99–1.14) (29). An older Cochrane review in 2004 assessed the optimal technique and agent for pleurodesis in patients with MPE and concluded that the available evidence supported the use of talc as the sclerosant of choice and that thoracoscopic pleurodesis was the preferred technique of pleurodesis (30). This was also demonstrated by another systematic review where talc insufflation was associated with less recurrence risk than talc slurry (RR 0.21, 95% CI, 0.05–0.93) (31). In a landmark trial, Dresler et al. (32) compared the role of thoracoscopic talc insufflation to talc slurry in 482 patients with MPE. The authors did not note any difference in the study arms in the percentage of patients with successful pleurodesis at 1 month (78% vs. 71%). However, in the post-hoc analysis patients with primary lung or breast cancer were noted to have a higher rate of pleurodesis with talc insufflation compared to talc slurry via the chest tube (82% vs. 67%). Terra et al. randomized 60 patients with recurrent MPE to video-assisted thoracoscopic talc insufflation vs. talc slurry and noted that immediate partial lung expansion was more frequently noted in the thoracoscopy group (60% vs. 30%, P=0.027) but noted no differences in clinical outcomes (33). Clive et al. conducted a meta-analysis assessing pleurodesis strategy in patients with MPE and found that talc insufflation had the highest rank in terms of fluid control (34). The authors did acknowledge the high level of heterogeneity between trials and the lack of patient-reported outcomes in their review. A recently concluded open label, randomized control trial across 17 hospitals in the United Kingdom compared the use of talc insufflation during thoracoscopy with moderate sedation to bedside chest tube insertion with talc slurry and did not show any difference in the rate of pleurodesis failure at 90 days. However, the authors acknowledged that the study might have been underpowered to detect small but potentially important differences (35).
Based on the above-published studies, recent guidelines from the ERS/EACTS and ESMO suggest that thoracoscopic talc poudrage (via surgical video assisted thoracoscopy or medical thoracoscopy) may be slightly more effective than talc slurry via chest tube for pleurodesis in patients with MPE (Evidence Grade II, B) (8,9). However, the ATS/STS/STR clinical practice guidelines suggest the use of either talc poudrage or talc slurry in patients with symptomatic MPE and expandable lung (7).
Thoracoscopy with tunneled indwelling catheter placement (TIPC)
TIPC placement is an alternative to chemical pleurodesis that offers patients the ability to perform regular home drainage of pleural fluid. TIPC placement is also the treatment of choice in patients with trapped/non-expandable lung, failed pleurodesis or loculated effusion (recommended by ATS/STS/STR, ERS/EACTS, ESMO). Multiple trials in the recent years have reported improvement in dyspnea and quality if life with the use of TIPC (7-9). A systematic review of 19 studies reported symptomatic improvement in 96% of patients after TIPC insertion (36). The TIME2 trial randomized patients to TIPC vs. inpatient talc slurry via a chest tube and noted improvement in dyspnea with TIPC at 6 months, reduced length of stay and reduced requirement of further procedures although with increased adverse events (37) The AMPLE study similarly randomized patients to TIPC vs. talc slurry via a chest tube and showed that patients with TIPC had shorter hospital stay, and required less subsequent pleural interventions (38). At least 30% of the patients with MPE have a non-expandable lung (32). Since chemical pleurodesis is rarely performed in these patients and the use of TIPC is associated with decreased length of stay and improvement in symptoms, their use may be option of choice in patients with a non-expandable lung (7-9).
While TIPC can be placed under local anesthesia with ultrasound guidance, some situations may warrant placement of TIPC during a thoracoscopy (Figure 1). This is especially relevant in patients with symptomatic MPE who also need more tissue for molecular testing. TIPC can also be placed in patients undergoing a diagnostic thoracoscopy along with talc insufflation (with complete or partial lung re-expansion) or in patients undergoing thoracoscopy and noted to have a non-expandable lung intraoperatively. Some authors noted a high pleurodesis rate in patients who underwent thoracoscopy and TIPC, even without the use of chemical pleurodesis. Suzuki et al noted a spontaneous pleurodesis rate of 53% in patients undergoing TIPC in the thoracoscopy cohort vs. 28% in patients undergoing TIPC placement via standard technique. This was especially relevant in the subgroup of patients with loculated pleural effusion undergoing thoracoscopy with adhesiolysis and TIPC placement (pleurodesis rate of 67% vs. 21%) (39). Similarly, in another study by Schneider et al., a spontaneous pleurodesis rate of 58% was noted in patients with trapped lung (noted intraoperatively) undergoing thoracoscopy with TIPC placement. The median duration to catheter removal was approximately 11 weeks (40). This higher rate of auto-pleurodesis may be explained by the dry pleural space post procedure, ability to break adhesions during the thoracoscopy as well as by the pleural inflammation as a consequence of pleural space invasion (thoracoscopy incisions and pleural biopsies) (39,41).
In patients with suspected or known recurrent and symptomatic MPE, these authors perform TIPC insertion via thoracoscopy in the following two circumstances, irrespective of lung expandability: (I) concurrent need for pleural biopsies; and (II) complex pleural effusion, adhesions and loculations as demonstrated by chest computed tomography and ultrasonography.
Irrespective of the technique of TIPC placement, it is important to determine the optimal drainage regimen via the TIPC in these patients. The multicenter ASAP trial demonstrated that daily drainage compared to alternate day drainage in patients with TIPC resulted in higher rates of auto-pleurodesis (47% vs. 24% P=0.003) (42). Similar rates of spontaneous pleurodesis were also demonstrated in the AMPLE2 trial which compared daily drainage to symptom-based drainage (44.2 vs. 15.9% P=0.004) though no difference in dyspnea control was noted between the two groups (43). It is therefore relevant that the frequency of drainage be documented when assessing the rates of spontaneous pleurodesis in trials or quality improvement projects pertinent to TIPCs.
Thoracoscopy with tunneled indwelling catheter placement and talc insufflation
There is increasing body of evidence supporting the use of TIPC in patients with MPE and this has led to an increased interest to combine their use in patients undergoing thoracoscopy with pleurodesis. Reddy et al. (44) studied 30 patients who underwent thoracoscopy with talc insufflation and placement of TIPC in the same procedure. The median duration of hospitalization was 1.79 days and pleurodesis was successful in 92% of the patients. The TIPC was removed at a median duration of 7.54 days post intervention. Similarly, Boujaoude et al. (45) conducted a prospective observational study with 29 patients undergoing thoracoscopic pleurodesis and TIPC placement. Pleurodesis was successful in 92% of patients at 1 month. The median length of stay was 3 days and the median duration of TIPC placement was 6 days. While there is a lack of randomized control trials for this combined approach, this a feasible treatment alternative, especially for patients with MPE who need to undergo a diagnostic thoracoscopy or are not willing to have TIPC for a prolonged period of time.
In patients with suspected or known recurrent and symptomatic MPE with complete or partial lung expandability, these authors perform thoracoscopy with talc insufflation and TIPC insertion in the following circumstances: (I) concurrent need for pleural biopsies; (II) complex pleural effusion, adhesions and loculations as demonstrated by chest computed tomography and ultrasonography; (III) patient’s unwillingness to have a long-term TIPC.
Management algorithm (Figure 2)
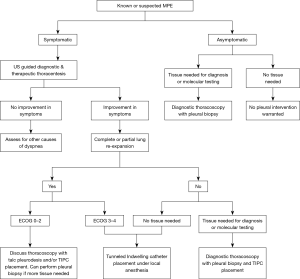
In patients suspected of having MPE, if the fluid cytology is negative for malignancy, thoracoscopy should be performed to obtain pleural biopsies. In patients with recurrent pleural effusions, a more definitive pleural intervention such as thoracoscopy with talc pleurodesis or placement of a TIPC should be offered. With the rapidly evolving targeted therapy in various malignancies, there is often need for repeat biopsies to assess for possible targetable mutations. In our opinion, patients who require repeat biopsy and have pleural effusions; a diagnostic and therapeutic thoracoscopy with talc pleurodesis and/or TIPC placement represents a patient-centered approach. The question of performing combined pleurodesis and TIPC versus either alone in the same sitting should be individualized based on functional status, expected length of stay, predicted duration of TIPC as well as expected adverse events.
Conclusions
The last decade has seen significant advancements in the management of patients with MPE as reflected by the new ATS/STS/STR and ERS/EACTS guidelines. We believe future research should focus on methods of fastening the time to pleurodesis, evaluating patient-reported outcomes, defining factors affecting pleurodesis and clarifying the difference between true pleurodesis and disease control. Continued education and awareness are warranted to ensure that guideline consistent care is provided to these patients suffering from advanced malignancies.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Kassem Harris) for the series “Interventional Pulmonology” published in Journal of Thoracic Disease. The article did not undergo external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.57). The series “Interventional Pulmonology” was commissioned by the editorial office without any funding or sponsorship. S Murgu: Educational consultant for Olympus, Boston Scientific, Pinnacle Biologics, Cook and Johnson & Johnson. A Agrawal has no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Musani AI, Haas AR, Seijo L, et al. Outpatient management of malignant pleural effusions with small-bore, tunneled pleural catheters. Respiration 2004;71:559-66. [Crossref] [PubMed]
- Grosu HB, Molina S, Casal R, et al. Risk factors for pleural effusion recurrence in patients with malignancy. Respirology 2019;24:76-82. [Crossref] [PubMed]
- Clive AO, Kahan BC, Hooper CE, et al. Predicting survival in malignant pleural effusion: development and validation of the LENT prognostic score. Thorax 2014;69:1098-104. [Crossref] [PubMed]
- Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J 2001;18:402-19. [Crossref] [PubMed]
- Ost DE, Niu J, Zhao H, et al. Quality Gaps and Comparative Effectiveness of Management Strategies for Recurrent Malignant Pleural Effusions. Chest 2018;153:438-52. [Crossref] [PubMed]
- Ost DE, Jimenez CA, Lei X, et al. Quality-adjusted survival following treatment of malignant pleural effusions with indwelling pleural catheters. Chest 2014;145:1347-56. [Crossref] [PubMed]
- Feller-Kopman DJ, Reddy CB, DeCamp MM, et al. Management of Malignant Pleural Effusions. An Official ATS/STS/STR Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198:839-49. [Crossref] [PubMed]
- Bibby AC, Dorn P, Psallidas I, et al. ERS/EACTS statement on the management of malignant pleural effusions. Eur Respir J 2018. [Crossref] [PubMed]
- Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2019;30:863-70. [Crossref] [PubMed]
- Prakash UB, Reiman HM. Comparison of needle biopsy with cytologic analysis for the evaluation of pleural effusion: analysis of 414 cases. Mayo Clin Proc 1985;60:158-64. [Crossref] [PubMed]
- Grosu HB, Kazzaz F, Vakil E, et al. Sensitivity of Initial Thoracentesis for Malignant Pleural Effusion Stratified by Tumor Type in Patients with Strong Evidence of Metastatic Disease. Respiration 2018;96:363-9. [Crossref] [PubMed]
- Agarwal R, Aggarwal AN, Gupta D. Diagnostic accuracy and safety of semirigid thoracoscopy in exudative pleural effusions: a meta-analysis. Chest 2013;144:1857-67. [Crossref] [PubMed]
- Dhooria S, Singh N, Aggarwal AN, et al. A randomized trial comparing the diagnostic yield of rigid and semirigid thoracoscopy in undiagnosed pleural effusions. Respir Care 2014;59:756-64. [Crossref] [PubMed]
- Lee P, Folch E. Thoracoscopy: Advances and Increasing Role for Interventional Pulmonologists. Semin Respir Crit Care Med 2018;39:693-703. [Crossref] [PubMed]
- Chrysanthidis MG, Janssen JP. Autofluorescence videothoracoscopy in exudative pleural effusions: preliminary results. Eur Respir J 2005;26:989-92. [Crossref] [PubMed]
- Schönfeld N, Schwarz C, Kollmeier J, et al. Narrow band imaging (NBI) during medical thoracoscopy: first impressions. J Occup Med Toxicol 2009;4:24. [Crossref] [PubMed]
- Shafiq M, Sethi J, Ali MS, et al. Pleural Cryobiopsy: A Systematic Review and Meta-Analysis. Chest 2020;157:223-30. [Crossref] [PubMed]
- Jancovici R, Lang-Lazdunski L, Pons F, et al. Complications of video-assisted thoracic surgery: a five-year experience. Ann Thorac Surg 1996;61:533-7. [Crossref] [PubMed]
- Viallat JR, Rey F, Astoul P, et al. Thoracoscopic talc poudrage pleurodesis for malignant effusions. A review of 360 cases. Chest 1996;110:1387-93. [Crossref] [PubMed]
- de Campos JR, Vargas FS, de Campos Werebe E, et al. Thoracoscopy talc poudrage: a 15-year experience. Chest 2001;119:801-6. [Crossref] [PubMed]
- Cardillo G, Facciolo F, Carbone L, et al. Long-term follow-up of video-assisted talc pleurodesis in malignant recurrent pleural effusions. Eur J Cardiothorac Surg 2002;21:302-5; discussion 305-6. [Crossref] [PubMed]
- Trotter D, Aly A, Siu L, et al. Video-assisted thoracoscopic (VATS) pleurodesis for malignant effusion: an Australian teaching hospital's experience. Heart Lung Circ 2005;14:93-7. [Crossref] [PubMed]
- Kolschmann S, Ballin A, Gillissen A. Clinical efficacy and safety of thoracoscopic talc pleurodesis in malignant pleural effusions. Chest 2005;128:1431-5. [Crossref] [PubMed]
- Arapis K, Caliandro R, Stern JB, et al. Thoracoscopic palliative treatment of malignant pleural effusions: results in 273 patients. Surg Endosc 2006;20:919-23. [Crossref] [PubMed]
- Steger V, Mika U, Toomes H, et al. Who gains most? A 10-year experience with 611 thoracoscopic talc pleurodeses. Ann Thorac Surg 2007;83:1940-5. [Crossref] [PubMed]
- Janssen JP, Collier G, Astoul P, et al. Safety of pleurodesis with talc poudrage in malignant pleural effusion: a prospective cohort study. Lancet 2007;369:1535-9. [Crossref] [PubMed]
- Barbetakis N, Asteriou C, Papadopoulou F, et al. Early and late morbidity and mortality and life expectancy following thoracoscopic talc insufflation for control of malignant pleural effusions: a review of 400 cases. J Cardiothorac Surg 2010;5:27. [Crossref] [PubMed]
- Yim AP, Chan AT, Lee TW, et al. Thoracoscopic talc insufflation versus talc slurry for symptomatic malignant pleural effusion. Ann Thorac Surg 1996;62:1655-8. [Crossref] [PubMed]
- Mummadi S, Kumbam A, Hahn PY. Malignant pleural effusions and the role of talc poudrage and talc slurry: a systematic review and meta-analysis. F1000Res 2014;3:254. [Crossref] [PubMed]
- Shaw P, Agarwal R. Pleurodesis for malignant pleural effusions. Cochrane Database Syst Rev 2004.CD002916. [PubMed]
- Tan C, Sedrakyan A, Browne J, et al. The evidence on the effectiveness of management for malignant pleural effusion: a systematic review. Eur J Cardiothorac Surg 2006;29:829-38. [Crossref] [PubMed]
- Dresler CM, Olak J, Herndon JE 2nd, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest 2005;127:909-15. [Crossref] [PubMed]
- Terra RM, Junqueira JJM, Teixeira LR, et al. Is full postpleurodesis lung expansion a determinant of a successful outcome after talc pleurodesis? Chest 2009;136:361-8. [Crossref] [PubMed]
- Clive AO, Jones HE, Bhatnagar R, et al. Interventions for the management of malignant pleural effusions: a network meta-analysis. Cochrane Database Syst Rev 2016.CD010529. [PubMed]
- Bhatnagar R, Piotrowska HEG, Laskawiec-Szkonter M, et al. Effect of Thoracoscopic Talc Poudrage vs Talc Slurry via Chest Tube on Pleurodesis Failure Rate Among Patients With Malignant Pleural Effusions: A Randomized Clinical Trial. JAMA 2019. [Epub ahead of print]. [PubMed]
- Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med 2011;26:70-6. [Crossref] [PubMed]
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012;307:2383-9. [Crossref] [PubMed]
- Thomas R, Fysh ETH, Smith NA, et al. Effect of an Indwelling Pleural Catheter vs Talc Pleurodesis on Hospitalization Days in Patients With Malignant Pleural Effusion: The AMPLE Randomized Clinical Trial. JAMA 2017;318:1903-12. [Crossref] [PubMed]
- Suzuki K, Servais EL, Rizk NP, et al. Palliation and pleurodesis in malignant pleural effusion: the role for tunneled pleural catheters. J Thorac Oncol 2011;6:762-7. [Crossref] [PubMed]
- Schneider T, Reimer P, Storz K, et al. Recurrent pleural effusion: who benefits from a tunneled pleural catheter? Thorac Cardiovasc Surg 2009;57:42-6. [Crossref] [PubMed]
- Bertolaccini L, Viti A, Terzi A. Management of malignant pleural effusions in patients with trapped lung with indwelling pleural catheter: how to do it. J Vis Surg 2016;2:44. [Crossref] [PubMed]
- Wahidi MM, Reddy C, Yarmus L, et al. Randomized Trial of Pleural Fluid Drainage Frequency in Patients with Malignant Pleural Effusions. The ASAP Trial. Am J Respir Crit Care Med 2017;195:1050-7. [Crossref] [PubMed]
- Muruganandan S, Azzopardi M, Fitzgerald DB, et al. Aggressive versus symptom-guided drainage of malignant pleural effusion via indwelling pleural catheters (AMPLE-2): an open-label randomised trial. Lancet Respir Med 2018;6:671-80. [Crossref] [PubMed]
- Reddy C, Ernst A, Lamb C, et al. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest 2011;139:1419-23. [Crossref] [PubMed]
- Boujaoude Z, Bartter T, Abboud M, et al. Pleuroscopic Pleurodesis Combined With Tunneled Pleural Catheter for Management of Malignant Pleural Effusion: A Prospective Observational Study. J Bronchology Interv Pulmonol 2015;22:237-43. [Crossref] [PubMed]


