
Left ventricular assist device-associated infections: incidence and risk factors
Introduction
Left ventricular assist device (LVAD) improves the survival of patients with severe end-stage heart failure under optimal medical therapy (1). The continuing development of long-term mechanical circulatory support over the last few decades has allowed the use of LVAD in bridge to transplant (BTT) or in destination therapy (DT) for selected patients. LVAD support is still associated with important adverse events that can lead to death (2). LVAD-associated infections are major complications and are associated with increased morbidity and mortality (3). Cellular immunity seems to be compromised by long-term LVAD support (4), which renders these patients more susceptible to bacterial and fungal infections (5).
LVAD-associated infections are the second most common adverse event after bleeding during the first 3 months after implantation (3). With extended LVAD support time, the risk of LVAD-associated infection increases (6,7). A study based on the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) registry revealed a significant number of percutaneous driveline infections within 8 months after implantation. In this study, age (<50 years) was the only predicting factor (8). The authors assumed a higher activity for patients aged <50 years than compared to that of the others, leading to increased trauma at the driveline exit site. Several others risk factors were identified, including older age, diabetes, larger body mass index, renal failure, malnutrition, and prolonged LVAD support (9-18).
However, data remain limited regarding risk factor analysis of LVAD-associated infections. The aim of this study was to assess the incidence of and to determine the risk factors for LVAD-associated infections.
Methods
Study population and data collection
This study was a retrospective analysis of all consecutive patients undergoing LVAD implantation between January 1, 2010, and January 1, 2019, at our institution. A total of 72 patients were enrolled in this single-center, observational study. For all consecutive patients operated on in our department, preoperative, intraoperative and postoperative data were systematically and prospectively collected in a database (ASTR, Access, Microsoft®). Infection-related data were retrospectively collected by review of patient’s medical files. Because, the study is a retrospective analysis of institutional data, and in compliance with the French law on clinical research, we only had to obtain French personal data protection authorization. We submitted our study to the national commission for data protection (CNIL) and obtained authorization. The study was approved by institutional ethic committee of the University Hospitals of Strasbourg.
Prevention of infection
Antimicrobial prophylaxis was systematically used. According to our institutional protocols, patients received 1.5 g of cefuroxime by intravenous injection and 0.75 g at priming, with a reinjection of 0.75 g every 2 h during the surgical operation. In case of allergy, vancomycin was used at 15 mg/kg/h. During the postoperative course, and the dressing change protocol for the driveline exit site was the same for all patients. The driveline exit site was cleaned every 2 days with an antiseptic combination of chlorhexidine, alcohol and benzalkonium chloride. Stabilization of the driveline was systematically used. A specialized nurse provided patient education after LVAD implantation.
LVAD surgery
A standardized procedure was used for LVAD implantation. The thoracotomy approach was started in 2016 and included two approaches: ministernotomy plus left anterolateral thoracotomy or left posterolateral thoracotomy. In conjunction with the thoracotomy approach, a double tunnel driveline technique was used with an incision under the left costal rim and an incision at the level of the right oblique muscle at the level of the umbilicus with the placement of the driveline behind the sheath. The velour part of the driveline was placed inside the body.
Patient follow-up
All patients were systematically followed during LVAD support in the Cardiac Surgery Department every month or every two months. Patients were followed-up until heart transplant or death. No patients were lost to follow-up. The median follow-up for the entire cohort was 1.3 (0.1–3.2) years.
Study endpoints
We used the definition for LVAD infections published by the International Society for Heart and Lung Transplantation (ISHLT) (19). According to this classification, infections were classified into three categories: percutaneous driveline infections, pocket infections and pump and/or cannula infections. The LVDA-associated infections group was defined as patients with at least one of these 3 categories of LVAD infections.
For each characterized infection, microbiological samples were documented. For each event, we indicated whether hospitalization was required and whether sepsis was related as well as the microorganisms found in the samples.
Statistical analysis
Continuous data are presented as the mean and standard deviation (SD) or the median and interquartile range (Q1–Q3). Categorical variables are presented as the number and percentage. Missing values were not imputed. We used Fisher’s exact test and the chi-square test to compare categorical variables. Continuous variables were compared with a t test. A P value less than 0.05 was considered statistically significant. We performed univariate and multivariate survival analyses, including covariates with a P value <0.05, using proportional hazards models. To check the proportional hazards assumption, we used the “Log of the negative log of the estimated survival function” (LoglogS) for categorial variables and the Schoenfeld residuals for continuous variables. The covariate “hypertension” did not respect this hypothesis and had no obvious link with the occurrence of infection. Thus, we removed this variable from the analysis. We considered heart transplant and death as competing events to analyze the occurrence of LVAD-associated infections. We used Cox regression to model the cause-specific hazard ratio (csHR) and the Fine and Gray model to estimate the subdistribution hazard ratio (sdHR) with the 95% confidence interval (CI). The analysis was stratified according to the indication for LVAD support (DT or BTT). To assess the probability of LVAD-specific infections, we estimated the cumulative incidence function (CIF). For each model, the Wald test was used to evaluate the hazards ratios. We used the PHREG procedure of SAS® 9.4 (SAS Institute, Inc., Cary, NC, USA) software for analysis.
Results
Preoperative characteristics
Preoperative variables sorted by the occurrence of LVAD-associated infections are reported in Table 1. The overall mean age was 59.8±13.3 years. Ischemic cardiomyopathy (46%) was the leading etiology for LVAD implantation. Most patients (52.8%) were either INTERMACS profile 1 (30.6%) or profile 2 (22.2%). The indications for LVAD implantation were BTT for 43 patients (59.7%) and DT for 29 patients (40.3%). Diabetes mellitus and hypertension were significantly more frequent in the control group than in the LVAD-associated infections group (P=0.034 and P=0.009, respectively).
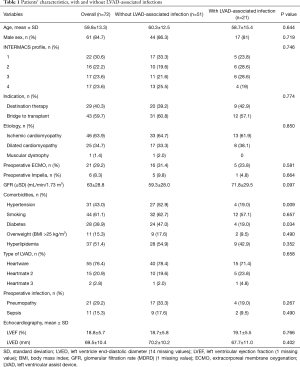
Full table
Operative and postoperative characteristics
Table 2 presents operative and postoperative characteristics. The median intensive care unit length of stay was 14 (Q1–Q3: 6–30) days. The median hospital length of stay after implantation was significantly higher in the LVAD-associated infections group than in the control group (P=0.036). More patients without LVAD-associated infections required postoperative hemodialysis (P=0.008).
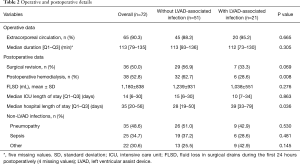
Full table
LVAD-associated infections
During the study period, 21 patients (29.2%) presented with a total of 32 LVAD-associated infections during their follow-up. Eight (38%) patients had more than one infection. Five (62.5%) pocket infections and one (50%) pump and/or cannula infection were preceded by a driveline infection. These infections are described in Table 3.
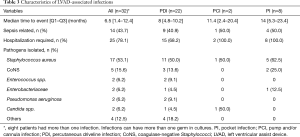
Full table
Percutaneous driveline infections represented 68.7% of all LVAD-associated infections. These infections occurred with a median delay of 8 (Q1–Q3: 4.8–10.2) months. Sepsis was associated with 40.9% of these infections. Percutaneous driveline infections required hospitalization in 68.2% of cases. Staphylococcus aureus and coagulase-negative staphylococci (CoNS) were responsible for 50.0% and 13.6%, respectively, of percutaneous driveline infections. Candida spp. was responsible for one (4.5%) percutaneous driveline infection.
Pocket infections and pump and/or cannula infections represented 25.0% and 6.3%, respectively, of LVAD-specific infections. Staphylococci were involved in 87.5% of pocket infections. Candida spp. was responsible for one (50.0%) pump and/or cannula infection.
The cumulative incidence function for LVAD-associated infections is presented in Figure 1. The probability of having a LVAD-associated infection at one year after implantation was 26.6% (95% CI: 17.5–40.5).
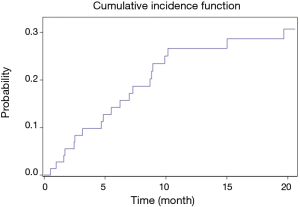
At the end of the study, 23.6% (n=17) of patients were diagnosed as being colonized with multidrug-resistant (MDR) bacteria. This colonization was more frequent in patients with LVAD-associated infections than in patients without LVAD-associated infections (42.9% vs. 15.7%; P=0.013). Extended-spectrum beta-lactamases (ESBLs) were involved in 94.1% (n=16) of cases of colonization with MDR bacteria. Only one patient was colonized with vancomycin-resistant enterococci (VRE).
Impact of the covariates
Following the comparison between groups with and without LVAD-specific infections, diabetes mellitus (P=0.034), hospital length of stay after implantation (P=0.036) and postoperative hemodialysis (P=0.008) (Tables 1,2) were analyzed via univariate and multivariate analyses. The non-respect of the proportional hazard assumption did not allow the use of hypertension in Cox or Fine and Gray models.
The results of the univariate analysis are presented in Table 4. Considering the competitive events, none of these covariates had a statistically significant impact on infection. However, hospital length of stay after implantation (csHR =0.48 per 10 days; 95% CI: 0.31–0.66; P<0.001) and postoperative hemodialysis (csHR =3.08; 95% CI: 1.34–7.08; P=0.007) were statistically correlated with death.
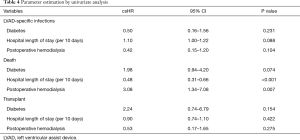
Full table
The results of the multivariate analysis are shown in Table 5. Hospital length of stay was statistically associated with infection (sdHR =1.22 per 10 days; 95% CI: 1.10–1.34; P=0.001) and death (sdHR =0.48 per 10 days; 95% CI: 0.25–0.90; P=0.019). Postoperative hemodialysis was statistically associated with a lower rate of LVAD-associated infections (sdHR =0.17; 95% CI: 0.05–0.57; P=0.004) and a higher rate of death (sdHR =5.23; 95% CI: 2.00–13.62; P<0.001).
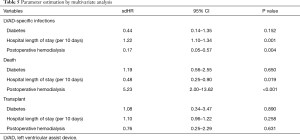
Full table
Discussion
Our study demonstrates that: (I) LVAD-associated infections are frequent, with a probability of having a LVAD-associated infection at 1 year after implantation of 26.6% and a median delay of occurrence of 6.5 months; (II) most of these infections are localized at the level of the driveline; (III) the risk factors of LVAD-associated infections according to multivariate analysis are a longer hospital length of stay and lower incidence of postoperative hemodialysis, which are strongly correlated to in-hospital mortality; and (IV) the major pathogens involved in LVAD-associated infections are S. aureus and CoNS.
The incidence of LVAD-associated infections in our study is similar to that reported in the literature (18,20-23). In our study, we observed a higher proportion of patients with an INTERMACS profile 1 under short-term mechanical support before LVAD implantation than that reported in the literature (22-24). This high incidence of critically ill patients was associated with a significant proportion of patients with preoperative infections. Despite these findings, we did not observe a higher incidence of LVAD-associated infections compared to what has been reported in others studies (20,21,24). Percutaneous driveline infections are the most frequent LVAD-associated infections, followed by pocket infections. Percutaneous driveline infection remains a significant complication because sepsis is often associated with this infection and frequently requires hospitalization. The European Association for Cardio-Thoracic Surgery has recently published an expert consensus on long-term mechanical circulatory support (1). Several recommendations have been issued regarding the prevention and treatment of infections before and after LVAD implantation (1). It must be stressed that most of these recommendations are part of our practice. Furthermore, as described elsewhere, we used the double tunnel technique for the placement of the driveline with a similar rate of LVAD-associated infections (23).
In the literature, age at implantation (8), diabetes mellitus (11-14) and a larger body mass index (9,12,17) are described as risk factors for infections. A recent publication of the ASSIST-ICD study identified the type of LVAD and patients who required implantable cardioverter defibrillator-related procedures post-LVAD as risk factors for LVAD-associated infections (20). In our study, there were no significant differences among these covariates between the two groups, with and without LVAD-associated infections. Only one patient in our study required implantable cardioverter defibrillator-related procedures post-LVAD. We found that postoperative hemodialysis was associated with a smaller incidence of LVAD-associated infections. Hospital length of stay was associated with an increase in infection. These results must be interpreted considering death. Major adverse events occur in the first 3 months after LVAD implantation and have an important effect on survival (3). Thus, we can consider that postoperative hemodialysis has a much greater impact on death than on the occurrence of LVAD-associated infections. Hospital length of stay after LVAD implantation may reflect survival. Likewise, acute kidney injury is a common postoperative complication of cardiac surgery and is associated with a high hospital mortality rate (25). The estimation of postoperative hemodialysis for the risk of LVAD-associated infections is biased by patients’ survival. Therefore, we can assume that patients who received postoperative hemodialysis and had a LVAD-associated infection were survivors of multiple postoperative complications.
Among the covariates, postoperative hemodialysis is an important predictor of death but does not have a clear impact on LVAD-associated infections.
Gram-positive cocci are the main pathogens isolated from microbiological samples. The bacteria of the digestive flora and Pseudomonas aeruginosa are also involved in LVAD-associated infections. These results agree with the literature (18). Most of these pathogens are susceptible to the antimicrobial prophylaxis (cefuroxime or vancomycin) used before operation. Fungi can also be responsible for LVAD-related infections (5), as confirmed in our study, with two cases of infection with Candida spp. being observed. Fungal infections are more complicated to manage and require heart transplant to have any chance for eradication of the infection.
The MDR bacteria colonization results are interesting. MDR colonization was more important in the LVAD-associated infections group than in the control group (42.9% vs. 15.7%; P=0.013). One explanation for this result may be the greater use of antibiotics in the LVAD-associated infections group. This information requires specific attention because it can be a risk factor for infection-associated death for patients with LVAD support (26).
We can note several limitations in our study. First, the retrospective review of medical files did not permit us to perform a standardized investigation. However, all patients were systematically followed in the Cardiac Surgery Department every month or every 2 months. Infection data were reviewed by two professionals separately, one of whom was an Infectious Disease specialist. Second, we analyzed a single-center cohort with a relatively small number of subjects. Third, we only focused on the incidence of and risk factors for LVAD-associated infections. The outcomes of LVAD-associated infections were not considered in our study. Lastly, some factors that were not measured may have acted as confounders.
Conclusions
LVAD-associated infections are important complications and are mostly represented by percutaneous driveline infections. S. aureus and CoNS are the predominant bacteria in these infections. Patients with LVAD-associated infections are more frequently colonized by MDR bacteria. The impact of this colonization should be evaluated with a prospective study.
Acknowledgments
We thank Mrs. Marie Tyman Heinrich for her help in collecting data and M. François Lefebvre for his help in statistical analysis.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.26). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Institutional Review Board approved the study procedures.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Potapov EV, Antonides C, Crespo-Leiro MG, et al. 2019 EACTS Expert Consensus on long-term mechanical circulatory support. Eur J Cardiothorac Surg 2019;56:230-70. [Crossref] [PubMed]
- Adzic A, Patel SR, Maybaum S. Impact of adverse events on ventricular assist device outcomes. Curr Heart Fail Rep 2013;10:89-100. [Crossref] [PubMed]
- Kirklin JK, Pagani FD, Kormos RL, et al. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J Heart Lung Transplant 2017;36:1080-6. [Crossref] [PubMed]
- Kimball PM, Flattery M, McDougan F, et al. Cellular immunity impaired among patients on left ventricular assist device for 6 months. Ann Thorac Surg 2008;85:1656-61. [Crossref] [PubMed]
- Bagdasarian NG, Malani AN, Pagani FD, et al. Fungemia associated with left ventricular assist device support. J Card Surg 2009;24:763-5. [Crossref] [PubMed]
- Sharma V, Deo SV, Stulak JM, et al. Driveline infections in left ventricular assist devices: implications for destination therapy. Ann Thorac Surg 2012;94:1381-6. [Crossref] [PubMed]
- Zierer A, Melby SJ, Voeller RK, et al. Late-Onset Driveline Infections: The Achilles’ Heel of Prolonged Left Ventricular Assist Device Support. Ann Thorac Surg 2007;84:515-20. [Crossref] [PubMed]
- Goldstein DJ, Naftel D, Holman W, et al. Continuous-flow devices and percutaneous site infections: clinical outcomes. J Heart Lung Transplant 2012;31:1151-7. [Crossref] [PubMed]
- John R, Holley CT, Eckman P, et al. A Decade of Experience With Continuous-Flow Left Ventricular Assist Devices. Semin Thorac Cardiovasc Surg 2016;28:363-75. [Crossref] [PubMed]
- Trachtenberg BH, Cordero-Reyes A, Elias B, et al. A review of infections in patients with left ventricular assist devices: prevention, diagnosis and management. Methodist DeBakey Cardiovasc J 2015;11:28-32. [Crossref] [PubMed]
- Monkowski DH, Axelrod P, Fekete T, et al. Infections associated with ventricular assist devices: epidemiology and effect on prognosis after transplantation. Transpl Infect Dis 2007;9:114-20. [Crossref] [PubMed]
- Robinson PJ, Billah B, Leder K, et al. Factors associated with deep sternal wound infection and haemorrhage following cardiac surgery in Victoria. Interact Cardiovasc Thorac Surg 2007;6:167-71. [Crossref] [PubMed]
- John R, Aaronson KD, Pae WE, et al. Drive-line infections and sepsis in patients receiving the HVAD system as a left ventricular assist device. J Heart Lung Transplant 2014;33:1066-73. [Crossref] [PubMed]
- Simon D, Fischer S, Grossman A, et al. Left ventricular assist device-related infection: treatment and outcome. Clin Infect Dis 2005;40:1108-15. [Crossref] [PubMed]
- Malani PN, Dyke DBS, Pagani FD, et al. Nosocomial Infections in Left Ventricular Assist Device Recipients. Clin Infect Dis 2002;34:1295-300. [Crossref] [PubMed]
- Mano A, Fujita K, Uenomachi K, et al. Body mass index is a useful predictor of prognosis after left ventricular assist system implantation. J Heart Lung Transplant 2009;28:428-33. [Crossref] [PubMed]
- Raymond AL, Kfoury AG, Bishop CJ, et al. Obesity and left ventricular assist device driveline exit site infection. ASAIO J 2010;56:57-60. [Crossref] [PubMed]
- Kusne S, Mooney M, Danziger-Isakov L, et al. An ISHLT consensus document for prevention and management strategies for mechanical circulatory support infection. J Heart Lung Transplant 2017;36:1137-53. [Crossref] [PubMed]
- Hannan MM, Husain S, Mattner F, et al. Working formulation for the standardization of definitions of infections in patients using ventricular assist devices. J Heart Lung Transplant 2011;30:375-84. [Crossref] [PubMed]
- Tattevin P, Flécher E, Auffret V, et al. Risk factors and prognostic impact of left ventricular assist device–associated infections. Am Heart J 2019;214:69-76. [Crossref] [PubMed]
- Hannan MM, Xie R, Cowger J, et al. Epidemiology of infection in mechanical circulatory support: A global analysis from the ISHLT Mechanically Assisted Circulatory Support Registry. J Heart Lung Transplant 2019;38:364-73. [Crossref] [PubMed]
- Kirklin JK, Xie R, Cowger J, et al. Second annual report from the ISHLT Mechanically Assisted Circulatory Support Registry. J Heart Lung Transplant 2018;37:685-91. [Crossref] [PubMed]
- Wert L, Hanke JS, Dogan G, et al. Reduction of driveline infections through doubled driveline tunneling of left ventricular assist devices-5-year follow-up. J Thorac Dis 2018;10:S1703-10. [Crossref] [PubMed]
- Stulak JM, Davis ME, Haglund N, et al. Adverse events in contemporary continuous-flow left ventricular assist devices: A multi-institutional comparison shows significant differences. J Thorac Cardiovasc Surg 2016;151:177-89. [Crossref] [PubMed]
- Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 2005;294:813-8. [Crossref] [PubMed]
- Papathanasiou M, Pohl J, Jánosi RA, et al. Colonization With Multiresistant Bacteria: Impact on Ventricular Assist Device Patients. Ann Thorac Surg 2018;105:557-63. [Crossref] [PubMed]


