
Uniportal versus multiportal video-assisted thoracic surgery for lung cancer: safety and advantages in employing complementary intraoperative lung ultrasound
Introduction
Surgery with radical intent is still the cornerstone of early stage non-small cell lung cancer therapy and anatomical lobectomy (followed by sampling or dissection of mediastinal lymph nodes) is regarded as the current surgical standard therapy. This recommendation is based on the evidence of decreased local recurrence and increased long-term survival in patients who underwent lobectomy compared with those subjected to wedge resection or segmentectomy (1). Limited resections have, therefore, to be reserved only for patients with poor performance status.
Current evidences suggest that Video-Assisted Thoracic Surgery (VATS) is a suitable approach for early-stage NSCLC lobectomy in terms of survival, local control of cancer and safety, when compared with open surgery (2). There is no standardized technique for the VATS approach: the number of incisions, or ports, may vary from two to four. The most common VATS lobectomy technique employed is the triportal approach according to Hansen et al. (3), which consists in two 1–1.5 cm lower access incisions—typically located in the 7th or 8th intercostal space, in the posterior and anterior axillary line respectively—for 2 thoracoscopic ports and a 4–5 cm port incision—placed in the 4th intercostal space, in the anterior axillary (in correspondence of the anterior margin of the latissimus dorsi muscle)—for a minithoracotomy (utility incision).
A single-port or “uniport” approach, performed with only one access, has been described in a number of recent papers. In lung cancer patients, both a triportal and a uniportal approach seem to reduce post-operative complications, such as pain, functional impairment and mortality, when compared to open surgery, furthermore showing a decreased operation time and intraoperative hemorrhage (4-6). However, close examination of the existing evidence comparing clinical outcomes of uniportal and multiportal VATS in the treatment of lung cancer has shown conflicting results.
Uniportal approach for VATS in lung cancer
In order to evaluate the safety and efficacy of an uniportal approach in VATS lobectomy, as a part of the surgical treatment protocol of lung cancer, Al-Ameri et al. (7) retrospectively analyzed early post-operative complications and outcomes in 333 patients. One hundred and twenty-two patients underwent uniportal VATS and 211 were subjected to a multiportal approach. Multiportal VATS was performed as a triportal anterior approach, according to the procedure described by Hansen et al. (3) or as a biportal anterior approach (excluding the posterior axillary access). The uniportal approach consisted in a single 4–5 cm incision in the 5th intercostal space, in the mid-anterior axillary line, as described by Gonzalez-Rivas et al. (8,9) with the camera held by the assistant surgeon in the posterior part of the incision. According to this study results, uniportal VATS lobectomy was safe in patients with lung cancer and showed an advantage in terms of a faster recovery stay after surgery compared to a multiportal VATS approach.
Comments and future perspectives
The aim in the development of minimally invasive surgery technique is to reach the right balance between optimal oncological radicality and the least possible invasiveness. In time, growing evidence suggested that, in patients with early stage NSCLC, the conventional triportal approach for VATS lobectomy, when associated with the appropriate lymph node dissection, was a good therapeutic approach and surgeons have successfully adopted and developed this technique over the past two decades. In recent years, near the increasing in number of VATS lobectomy performed, there is also the attempt to reduce to a minimum the number of port incisions. In particular, the possibility to employ an uniportal approach has been generating an increasing interest over the past few years (8,10). Since 2011, the use of uniportal VATS instead of open surgery has shown to be a safe and effective technique with outcomes similar to those of multiportal approaches. A claimed advantage of the uniportal approach for the surgeon is to allow the use of parallel instrumentation and bimanual work, providing a more direct and anatomical view of the target tissues, as in an open surgery. Nevertheless, due to its distinct advantage of requiring only a single incision, uniportal approach is expected to minimize the amount of surgical trauma, and this may reflect in decreased post-operative functional impairment and pain, promoting more rapid recovery when compared with pulmonary lobectomy via the traditional triportal approach. In a recent systematic review uniportal VATS was associated to improved early post-operative outcomes compared to multiportal VATS, such as reduction in hospital stay, need of post-operative chest tube drainage, blood loss and mortality. In the same meta-analysis no significant differences were found between the two approaches in regard to peri-operative outcomes, such as number of lymph nodes dissected, operative time and rates of conversion to open thoracotomy (11). Results from the study of Al-Ameri et al. (7) confirmed that uniportal VATS lobectomy is feasible and safe when introduced in the treatment program of lung cancer patients and suggested that uniportal VATS approach might shorten the needed recovery stay after surgery.
However, not all the currently published literature agrees with the assumption that the uniportal approach may hold advantages over the multiportal one. Indeed controversial results are reported both in terms of post-operative and peri-operative outcomes (12). Nevertheless, long-term clinical outcomes were not assessed in any of the studies comparing the two VATS methods, thus being not possible to compare oncologic efficacy over time. On this background it seems premature to affirm the superiority of the uniportal technique on the multiportal one.
In a Canadian single-centre single-surgeon retrospective study no differences were found regarding intra-operative and post-operative complications between uniportal and multiportal VATS approach, but a slightly higher conversion rate to open thoracotomy was recorded in the uniportal group (13). This phenomenon may be explained by the fact that the multiportal VATS approach has been safely used and implemented for more than 20 years and it is logical that most thoracic surgeons are more confident with this technique, even in high-volume thoracic surgery centers.
During the years multiportal VATS techniques have evolved (and continues to do it), also according to technical advances in imaging techniques and the perfection of surgical instruments. As a result, multiportal VATS has gained a role also in the diagnostic and therapeutic iter of peripheral solitary or multiple lung nodules (14,15).
Minimally invasive procedure, such as transthoracic or transbronchial biopsy, are considered as the method of choice for the etiological clarification of a lung nodule. The employment of the one or the other method depends on the size and location of the target lesion. Surgical resection of the entire nodule in VATS, followed by histologic examination, allows simultaneously the precise diagnosis and the rapid therapy of an eventual lung cancer in early stage. In addition, video-thoracoscopy is a very useful staging procedure for lung cancer, allowing a full visualization of the pleural space, including the assessment of both the parietal and the visceral pleura and all ipsilateral nodal regions.
VATS procedures’ main limit is that palpation of the lung surface is not always possible and, in cases of smaller or deeper lesions, we need to employ additional techniques in order to discover the nodule(s) (16). In this context, intra-operatory lung ultrasound (ILU) is a complementary method of localization that may allow to identify, safely and in real-time, also smaller nodules or structures that can’t be visualized by other preoperative imaging methods. It could be considered as the corresponding to Endobronchial Ultrasound (EBUS) for the histological assessment of subpleural lesions.
This technique was developed for a multiportal VATS approach (triportal or biportal) with at least one thoracoscopic and one operative access, but it could be suitable also for uniportal approach (17,18). However, if we choose to perform ILU under uniportal approach, we have to renounce to the access for the direct consensual optical view and this could lead to problems, such as accidental/involuntary lung insults, because we have not, at the same time, the possibility to clearly see where the instrument is going.
Recently we applied ILU to the study of patients with different interstitial lung diseases and/or suspected lung nodules and indication for diagnostic VATS (19,20). After the procedure, all patients were subjected to a pre-operative chest imaging, including high-resolution computed-tomography (HRCT), contrast-enhanced computed-tomography (CT), and/or 18F-FDG positron emission tomography integrated with computed tomography (PET-TC). In addition, patients underwent to a transthoracic ultrasound (TUS) examination in order to record the pattern of the lesion(s) of interest and compare it with ILU findings. Wedge resection of all the nodules detected in ILU examination was performed. The final histological diagnoses of lung nodules in our cohort were cancer (adenocarcinoma and squamous carcinoma), benign lesion (hamartochondroma) and histiocytosis X.
The main difference between TUS and ILU approach is that the second technique is not limited by differences in chest wall/pulmonary air content acoustic impedance, being the probe directly placed on the lung (21). In healthy parts of lung the only ILU finding was a thin hyperechoic line, due to the interface between the US beam, the saline solution (with which the pleural space was filled in order to obtain a better ILU view) and the lung parenchyma, in the absence of artifacts. Interestingly, in fibrotic lung ILU examination showed a thickening of the hyperechoic pleural line without artifact below, while the TUS had previously shown the presence of B-lines artifacts underlying an irregularly thickened pleural line. Such evidence can be explained by the fact that ultrasound generation of artifacts (B-lines and A-lines) is due only to the high difference in acoustic impedance between the different structures crossed by the US beam in transthoracic approach and shouldn’t, therefore, be used as an unequivocal ultrasound marker of pathology.
In one patient with a pulmonary nodule detected by HRCT, ILU allowed to identify a second smaller nodule (3.5 mm in diameter) located nearby the nodule to be resected but that has been not possible to asses with pre-operative HRTC and bimanual palpation during VATS. Histological examination of such nodule revealed a final diagnosis of adenocarcinoma.
Despite it’s not possible to distinguish between malignant and benign lesions on the basis of the ultrasound pattern, data from our preliminary case report study have confirmed that intraoperative US allowed not only to visualize all the lesions previously assessed by TUS and chest HRCT, but also smallest lesions (of few millimeters) that is not possible to identify during the pre-operative studies (CT, PET) and that cannot be assessed during VATS through palpation. In addition, ILU showed the potential to allow the identification of the limits between the nodule(s) and the circumstantial lung parenchyma and, therefore, to guide minimally invasive lung resections during VATS (Figure 1).
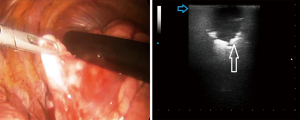
Therefore, we believe that intraoperative lung ultrasound (ILU) is another new technique that deserves to be developed in reason of its ability to effectively localize also invisible or non-palpable pulmonary nodules in real-time during VATS, helping surgeons to perform biopsies with clear surgical margins and higher histological diagnostic yield. In this context, it may be desirable to continue to employ the triportal VATS technique, which allows a more coherent and secure complementarity of the techniques (direct optic view plus real-time ultrasound assessment). Further comparative studies, better if prospective in design, wider in cases and with randomized patients’ assignation to the two compared groups of treatment, are needed in order to demonstrate if the uniportal approach significantly improves early and long-term post-operative outcomes, thus proving it as truly minimally invasive and effective even with respect to the triportal method. With this purpose, it would also be interesting to try to implement the uniportal technique with the use of an endoscope that has an optical vision and an ultrasound trasducer simultaneously combined.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.45). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ginsberg RJ, Rubinstein L V. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Ann Thorac Surg 1995;60:615-22. [Crossref] [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- Hansen HJ, Petersen RH. Video-assisted thoracoscopic lobectomy using a standardized three-port anterior approach - The Copenhagen experience. Ann Cardiothorac Surg 2012;1:70-6. [PubMed]
- Long H, Tan Q, Luo Q, et al. Thoracoscopic Surgery Versus Thoracotomy for Lung Cancer: Short-Term Outcomes of a Randomized Trial. Ann Thorac Surg 2018;105:386-92. [Crossref] [PubMed]
- Bendixen M, Licht PB. Video-assisted thoracoscopic surgery versus open lobectomy for lung cancer: Time for a randomized trial. Eur J Cardiothorac Surg 2017;52:398. [Crossref] [PubMed]
- Ismail M, Swierzy M, Nachira D, et al. Uniportal video-assisted thoracic surgery for major lung resections: Pitfalls, tips and tricks. J Thorac Dis 2017;9:885-97. [Crossref] [PubMed]
- Al-Ameri M, Sachs E, Sartipy UJV. Uniportal versus multiportal video-assisted thoracic surgery for lung cancer. J Thorac Dis 2019;11:5152-61. [Crossref] [PubMed]
- Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: Two years of experience. Ann Thorac Surg 2013;95:426-32. [Crossref] [PubMed]
- Hernandez-Arenas LA, Purmessur RD, Gonzalez-Rivas D. Uniportal video-assisted thoracoscopic segmentectomy. J Thorac Dis 2018;10:S1205-14. [Crossref] [PubMed]
- Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [Crossref] [PubMed]
- Harris CG, James RS, Tian DH, et al. Systematic review and meta-analysis of uniportal versus multiportal video-assisted thoracoscopic lobectomy for lung cancer. Ann Cardiothorac Surg 2016;5:76-84. [Crossref] [PubMed]
- Sihoe ADL. Uniportal Lung Cancer Surgery: State of the Evidence. Ann Thorac Surg 2019;107:962-72. [Crossref] [PubMed]
- Bin Yameen TA, Gupta V, Behzadi A. Uniportal versus multiportal video-assisted thoracoscopic surgery in the treatment of lung cancer: a Canadian single-centre retrospective study. Can J Surg 2019;62:468-74. [Crossref] [PubMed]
- DeCamp MM, Jaklitsch MT, Mentzer SJ, et al. The safety and versatility of video-thoracoscopy: a prospective analysis of 895 consecutive cases. J Am Coll Surg 1995;181:113-20. [PubMed]
- Kaiser LR. Video-assisted thoracic surgery: Current state of the art. Ann Surg 1994;220:720-34. [Crossref] [PubMed]
- Shennib H. Intraoperative localization techniques for pulmonary nodules. Ann Thorac Surg 1993;56:745-8. [Crossref] [PubMed]
- Rocco G, Cicalese M, La Manna C, et al. Ultrasonographic identification of peripheral pulmonary nodules through uniportal video-assisted thoracic surgery. Ann Thorac Surg 2011;92:1099-101. [Crossref] [PubMed]
- Rocco G, Rocco R, Scarci M. Intraoperative Ultrasound Guidance in Pulmonary Nodule Localization in Uniportal VATS. In: Atlas of Uniportal Video Assisted Thoracic Surgery. Springer, 2019:101-2.
- Frongillo E, Taurchini M, Del Colle A, et al. Transthoracic Ultrasound and Intraoperative Lung Ultrasound. Biomed J Sci Tech Res 2019. [Crossref]
- Sperandeo M, Frongillo E, Dimitri LMC, et al. Video-assisted thoracic surgery ultrasound (VATS-US) in the evaluation of subpleural disease: preliminary report of a systematic study. J Ultrasound 2020;23:105-12. [Crossref] [PubMed]
- Tinti MG, Frongillo E, Sperandeo M. Lung Fissures Detection With Transthoracic Ultrasound: Is It Simply a Matter of Artifacts? Chest 2018;154:453-5. [Crossref] [PubMed]


