
Primary pneumonectomy, pneumonectomy after induction therapy, and salvage pneumonectomy: a comparison of surgical and prognostic outcomes
Introduction
According to the annual report of the Japanese Association for Thoracic Surgery, 38,444 cases of surgery performed to treat pulmonary malignancies in 2014 in Japan included 27,584 (71.8%) cases of lobectomy and only 521 (1.4%) cases of pneumonectomy, indicating that pneumonectomy is a rare treatment modality used for lung cancer (1). Understandably, after induction chemoradiotherapy to treat locally advanced lung cancer, pneumonectomy is even more uncommon. Data from the World Conference on Lung Cancer show that 88% of 11,242 patients with IIIA-N2 non-small-cell lung cancer were treated with radical chemoradiotherapy, whereas 5% of these patients were treated with lobectomy after induction therapy, and pneumonectomy has been rarely used only in 1% of patients (2).
Although pneumonectomy after induction chemoradiotherapy may possibly be an inferior therapeutic strategy relative to radical non-operative chemoradiotherapy (3), contradicting positive results have also been obtained (4). Furthermore, pneumonectomy is performed very rarely as salvage resection after radical non-operative therapy for lung cancer (5-7). Positive outcomes reported after pneumonectomy (4) prompted us to reexamine all cases of pneumonectomy performed at our institution and to compare primary pneumonectomy with pneumonectomy after induction therapy and salvage pneumonectomy after radical chemoradiotherapy.
Methods
Study design and patients
This study was approved by the Institutional Review Board of the Aichi Cancer Center Hospital (approval No. 2017-1-330). Informed consent was obtained from each patient for the use of clinical data for various studies.
Among 3,989 patients who underwent pulmonary resection for lung cancer between 1990 and 2016 at our hospital, including those who underwent surgery after induction therapy (165 patients) and those who underwent salvage lung resection (18 patients), 151 consecutive patients who underwent pneumonectomy were examined. For retrospective analysis, these patients were divided into three groups: a primary pneumonectomy group (pneumonectomy without preoperative nonsurgical treatment, n=137), an induction group (pneumonectomy as planned pulmonary resection after certain preoperative treatment, n=10), and a salvage group (pneumonectomy defined as salvage surgery for a residual tumor after radical non-operative therapy or for enlarged lesions, n=4, Table 1). The induction group was defined as the group treated with ≥3 cycles of platinum-based chemotherapy or radical chemoradiotherapy. In the salvage group, tumor progression was comprehensively confirmed based on its enlargement observed on computed tomography, increased abnormal accumulation in lesions observed on positron emission tomography (PET), increased tumor marker levels, and other findings leading to pneumonectomy as salvage surgery wherein patients did not undergo any therapy other than surgery. Completion pneumonectomy was performed only in the primary pneumonectomy group (right, 2 patients; left, 6 patients) and was included in the study.
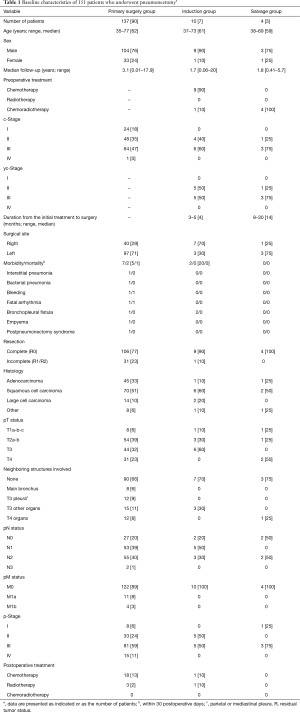
Full table
All patients were analyzed based on the eighth edition of the tumor-node-metastasis (TNM) staging classification system (8,9). In this study, the gross tumor size on pathological examination was used because reevaluation of the pathological invasive size of all tumors during the study period was difficult, and because the eighth edition for evaluating the lesion size was proposed in April 2016 after the proposal for a new classification in February 2016; cT and pT in the proposal of the eighth edition (8,9) and its validation (10) were still evaluated using the gross tumor size as done previously. The eighth edition affects the assessment of small lesions with a lepidic component to a greater extent; however, stage IB, II, and III tumors that were mainly examined in this study were considered solid lesions minimally affected by the method used for lesion size measurement compared with stage IA tumors. These points have been discussed previously (11). Completeness of resection was categorized to a complete (R0) or incomplete (R1/R2) resection based on the residual tumor status.
Pathological assessment
Pathological slides of resected lung specimens were prepared using a standard procedure. Briefly, the resected lung was inflated immediately and fixed by injection with 10% formalin. Sliced tissues were embedded in paraffin, and the blocks were sectioned and stained with hematoxylin and eosin. Elastin and immunohistochemical staining were performed when necessary. The histological effect (Ef.) of preoperative therapy was assessed using resection specimens on the following 5-point scale according to the General Rule for Clinical and Pathological Record of Lung Cancer (12): Ef.0, no effect, no morphological changes including degeneration or necrosis caused by treatment; Ef.1a, minor effect, viable cancer cells observed in two-thirds or more of cancer tissue; Ef.1b, mild effect, viable cancer cells observed in one-third or more and less than two-thirds of cancer tissue; Ef.2, moderate effect, viable cancer cells observed in less than one-third of cancer tissue; and Ef.3, marked effect, no viable cancer cells or residual cancer cells judged not to be viable.
Statistical analysis
The overall survival (OS) rate was calculated using the Kaplan-Meier method. OS was defined as the time from the date of surgery to that of all-cause death, and patients who were alive were censored on the last known date. Difference in survival rate was compared using the log-rank test and Cox proportional hazards model. All P values were two-sided, and P<0.05 was considered statistically significant. All statistical analyses were performed using JMP for Windows (version 9.0, SAS Institute, Cary, NC, USA).
Results
Patients’ demographics
The patients’ demographics and disease characteristics are summarized in Table 1. In the induction group, various chemotherapy regimens were used in three [cisplatin + vindesine + mitomycin], two (cisplatin + vinorelbine), one (cisplatin + docetaxel), three (carboplatin + docetaxel), and one (carboplatin + paclitaxel) patient, and one patient underwent chemoradiotherapy at a tumor bed boost dose of 60 Gy. In this group, postoperative pathological assessment of the resected specimens revealed that the Ef. obtained was mixed; Ef.0 was observed in three patients, Ef.1 in five patients (including one patient who underwent chemoradiotherapy), and Ef.2 in two patients; no Ef.3 was observed. Alternatively, all patients in the salvage group had a history of cisplatin-based chemotherapy and radical radiotherapy, followed by treatment with multiple chemotherapy regimens.
Mortality and morbidity
Severe perioperative complications, including interstitial pneumonia, bacterial pneumonia, fatal hemorrhage, fatal arrhythmia, bronchopleural fistula, and postpneumonectomy syndrome, were observed in 5.1% (7/137), 20% (2/10), and 0% (0/4) of patients in the primary pneumonectomy, induction, and salvage groups, respectively; these complications occurred frequently in the induction group but not in the salvage group. Among these, complication-related death within 30 postoperative days occurred in 1.5% (2/137) of patients in the primary pneumonectomy group but not in the induction and salvage groups. Death after 30 postoperative days but within 90 days occurred in 3.6% (3/137, cancer-related death), 10% (1/10, cancer-related death), and 0% (0/4) of patients in the primary pneumonectomy, induction, and salvage groups, respectively. In the induction group, severe morbidity occurred because of bronchopleural fistulas in two patients, one of whom was treated with chemoradiotherapy, although their bronchial stump was buttressed using an intercostal muscle flap. Although both patients were rescued by omentopexy, recurrence led to cancer-related death after 1.6 years in one patient and 1.8 years in the other patient.
Prognostic outcomes
The primary prognostic factors for all patients who underwent pneumonectomy are summarized in Table 2. Univariate analysis revealed that age, completeness of resection, tumor size, depth and sites of invasion of neighboring organs, and the pN factor were significant prognostic factors. The 3-year OS rate for patients without invasion of neighboring organs (n=100) was 54.8%, and those with infiltration only to the main bronchus or pleura (n=20) was 64.3%. Patients with pN2 disease showed significantly worse prognosis than those with pN0–1 diseases, but the significance decreased in multivariate analysis. Multivariate analysis revealed that completeness of resection (P=0.003) and subcategorization of involved neighboring organs (P=0.008) were more significant than the presence or absence of mediastinal lymph node metastasis (P=0.033) and were the crucial predictors in patients who underwent pneumonectomy. On the contrary, regarding the surgical site (right or left) of pneumonectomy, the prognosis tended to be slightly better for left side in this study, but failed to reach a statistically significant difference. Adjuvant therapy also seemed to be associated with better prognosis, but was not statistically significant.
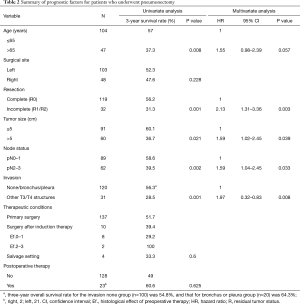
Full table
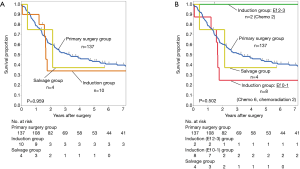
There was no significant prognostic difference between different therapeutic strategies, with the 3-year OS rate being 51.7%, 39.4%, and 33.3% in the primary pneumonectomy, induction, and salvage groups, respectively (Figure 1A, Table 2). When the patients in the induction group were stratified based on the Ef., the 3-year OS rate was 100% in the Ef.2–3 group and 29.2% in the Ef.0–1 group (P=0.24), indicating a longer survival of patients in the Ef.2–3 groups (Figure 1B, Table 2).
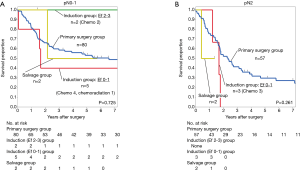
The main study results were obtained by stratifying the above findings based on pN factor-based subcategorization (Figure 2). Among patients with pN0–1 disease (Figure 2A), the 3-year OS rate was 58.7% (n=80) in the primary pneumonectomy group, 100% (n=2) for patients with Ef.2–3 in the induction group, 40% (n=5) for patients with Ef.0–1 in the induction group, and 50% (n=2) in the salvage group. Among patients with pN2 disease (Figure 2B), this rate was 41.4% (n=57) in the primary pneumonectomy group, and there were no patients with Ef.2–3 in the induction group. Three patients with Ef.0–1 in the induction group died of the underlying disease after 1.7 postoperative years. In the salvage group, one patient died of the underlying disease after 0.5 postoperative years, and another patient who survived had lung cancer recurrence. Among patients with pN2 disease, no patients in the induction and salvage groups survived to 2 years postoperatively.
Discussion
The possible prognostic factors after pneumonectomy reportedly include completeness of resection, presence or absence of mediastinal lymph node metastasis, and disease stage (13,14). Alexiou et al. summarized 206 pneumonectomies and reported that prognosis was poor in elderly patients and in those with a higher pathological stage, resulting in an operative mortality of 6.8% (13). Similarly, Licker et al. analyzed 193 pneumonectomies and reported 9.3% mortality within 30 postoperative days (14). In the present investigation, based on a similar sample size of patients, mortality within 30 postoperative days and that after 30 postoperative days but within 90 days was observed in 1.5% and 3.6% patients, respectively, in the primary pneumonectomy group.
Furthermore, we found that the prognostic outcome of pneumonectomy differs substantially based on the degree of invasion of neighboring organs. In cases with no invasion or infiltration only to the main bronchus or pleura, the outcome was significantly better than that in cases with the invasion of other deeper structures. As reported previously on the tumor invasion of neighboring organs (11,15), the invasion status of patients undergoing pneumonectomy may be a significant prognostic factor following completeness of resection, perhaps more significant than the presence or absence of mediastinal lymph node metastasis (Table 2).
The therapeutic strategy of performing pneumonectomy after induction therapy has revealed conflicting results. Martin et al. examined cases of various lung resection after induction therapy, including those who underwent pneumonectomy, and reported a mortality rate of 23.9% (11/46) following right pneumonectomy and an overall mortality of 11.3% (11/97) (16). Weder et al. reported excellent outcomes in 176 patients treated with pneumonectomy after induction therapy (chemotherapy, 20%; chemoradiotherapy, 80%) whereby the incidence of major complications was 13%, the 3-year OS rate was 43%, and the 5-year OS rate was 38% (4). In contrast, Albain et al. reported worse outcomes in patients treated with pneumonectomy than in those treated with radical chemoradiotherapy in a phase III study on chemotherapy plus radiotherapy followed by surgery in patients with IIIA-N2 lung cancer, leading to the conclusion that pneumonectomy cannot be recommended for these patients (3).
The choice between chemoradiotherapy and chemotherapy as induction treatment and the method to manage the radiotherapy dose have been controversial. Daly et al. reported a 5-year OS rate of only 33%, with no increase in the risk of mortality with right pneumonectomy in patients who underwent pneumonectomy (right, 18 patients; left, 12 patients) after high-dose radiation and concurrent chemotherapy; serious complications occurred in five patients, with perioperative deaths in four who underwent left pneumonectomy (17). Sonett et al. reported that a complete pathological response was achieved in 48% patients [lobectomy, 29 patients; pneumonectomy, 11 (right, 7; left, 4) patients] after high-dose radiation exceeding a dose of 59 Gy was given with concurrent chemotherapy (18). The authors emphasized that although surgery after high-dose radiation resulted in positive outcomes, bronchopleural fistulas can often form in patients with an uncovered bronchial stump and the incidence of complications can increase when using this therapeutic strategy in practice.
Conversely, it was claimed that radiotherapy did not have any additive effects on induction therapy in IIIA-N2 lung cancer (19-21). For 232 patients with IIIA-N2 lung cancer, Pless et al. compared surgery after induction chemoradiotherapy (117 patients including 25 who underwent pneumonectomy) with that after induction chemotherapy (115 patients including 19 who underwent pneumonectomy) and stated that one definitive local treatment modality combined with neoadjuvant chemotherapy was adequate to treat resectable stage IIIA-N2 non-small cell lung cancer (19). In a retrospective analysis conducted using the National Cancer Database that compared surgery after induction chemotherapy (528 patients including 83 who underwent pneumonectomy) and surgery after induction chemoradiotherapy (834 patients including 168 who underwent pneumonectomy), Yang et al. showed the non-inferiority of chemotherapy to chemoradiotherapy in terms of OS (20), and Shah et al. reported similar results in their meta-analysis (21).
Alternatively, very few reports have discussed salvage resection, and none have focused on salvage pneumonectomy. Bauman et al. examined the prognosis of 24 cases of salvage lung resection (including 10 cases of pneumonectomy) and stated that PET was effective for salvage surgery (5); this finding corroborated those reported by Schreiner et al., who reviewed nine recent salvage lung resection reports including some by Bauman et al. and concurred with the overall effectiveness of salvage resection (6). However, these reviews focused mainly on cases of salvage resection after stereotactic radiotherapy. In Japan, Uramoto et al. reported eight cases of salvage surgery, including one case of pneumonectomy (7). In the present cohort, no patient with pN2 disease treated with salvage pneumonectomy survived for 2 years postoperatively, indicating that when considering salvage pneumonectomy, it is crucial to proactively diagnose mediastinal lymph node metastasis preoperatively. Nevertheless, in critical situations wherein salvage surgery could be the only therapeutic choice, a decision to perform it on a case-by-case basis should be taken; if N2-positive lymph nodes are found, salvage surgery can be avoided or attempted despite poor prognosis because there are no other therapeutic options.
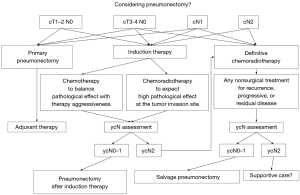
A review of all cases including the present investigation raises an important question of how to establish a daily clinical practice concerning pneumonectomy. Figure 3 shows the possible treatment strategy in our daily practice for patients who could be treated with pneumonectomy. Whether to use only chemotherapy while placing importance on the significance of induction therapy as a systemic treatment or to use chemoradiotherapy while aiming to obtain higher histological effect at the surgical site is a difficult decision. Obtaining better histological effects should be balanced with surgical aggressiveness. In daily practice, we prefer chemotherapy over chemoradiotherapy for induction treatment to surgery, particularly when pneumonectomy is considered for the following reasons: (I) if only chemotherapy is used as a neoadjuvant treatment, postoperative radical radiation dose can be adjusted according to disease progression or when irradiation becomes necessary; (II) some reports suggest that combining radiation with preoperative treatment is not effective regardless of the disease condition (cN1 or cN2) (19-21); (III) surgery may be generally more difficult, and more complications may occur after induction chemoradiotherapy than after induction chemotherapy (16-18); (IV) induction chemotherapy could lead to downstaging, making it possible to avoid pneumonectomy (19-21); however, this treatment strategy may not necessarily be effective and a surgery-first strategy may suffice for the cN1 disease condition (22); and (V) postoperative adjuvant chemotherapy is more difficult after pneumonectomy than after lobectomy; therefore, chemotherapy could be performed preoperatively. In the induction group in this study, as high Ef. tended to be attained in patients with well-differentiated cancer and Ef. tended to be low in patients with poorly differentiated to undifferentiated cancer, the histological differentiation of the tumor may be a factor that predicts the reaction to induction therapy (detailed data were not shown).
This study has many limitations. One consideration regarding the experimental design of this investigation is the small sample size of patients treated with pneumonectomy after induction therapy or salvage pneumonectomy compared with the more rigorous and extensive reports in the literature cited above. A retrospective analysis of data from a single institution and a long surveillance period also limit generalization of the present findings. Considering the recent advances in molecular targeted therapies and immune checkpoint inhibitors, the relevance of the findings may be outdated. However, the study objective was to compare the outcomes of rarely performed pneumonectomy after induction therapy and even rarely performed salvage pneumonectomy with primary pneumonectomy, which enables a comprehensive understanding of practical pneumonectomies performed at a cancer center in Japan.
The outcomes of pneumonectomy differ considerably when there is an absence of invasion of neighboring organs or infiltration only to the main bronchus or pleura, and when there is an invasion of other deeper structures. Despite being performed after radical chemoradiotherapy, salvage pneumonectomy was easier to perform with fewer severe complications than pneumonectomy after induction therapy. With pN2 disease, the 2-year survival seemed to be difficult to achieve in patients who experienced pneumonectomy in the induction and salvage settings. It could be speculated that when considering pneumonectomy, it is essential to balance the pursuit of histological effects with treatment aggressiveness in induction settings and to strictly select patients with pN0–1 disease for salvage settings.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.19). YS serves as an unpaid editorial board member of Journal of Thoracic Disease from Aug 2019 to Jul 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board of the Aichi Cancer Center Hospital (approval no. 2017-1-330). Informed consent was obtained from each patient for the use of clinical data for various studies.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Masuda M, Okumura M, Doki Y, et al. Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery. Thoracic and cardiovascular surgery in Japan during 2014: Annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2016;64:665-97. [Crossref] [PubMed]
- Koshy M, Fedewa SA, Malik R, et al. Improved survival associated with neoadjuvant chemoradiation in patients with clinical stage IIIA(N2) non-small-cell lung cancer. J Thorac Oncol 2013;8:915-22. [Crossref] [PubMed]
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379-86. [Crossref] [PubMed]
- Weder W, Collaud S, Eberhardt WE, et al. Pneumonectomy is a valuable treatment option after neoadjuvant therapy for stage III non-small-cell lung cancer. J Thorac Cardiovasc Surg 2010;139:1424-30. [Crossref] [PubMed]
- Bauman JE, Mulligan MS, Martins RG, et al. Salvage lung resection after definitive radiation (>59 Gy) for non-small cell lung cancer: surgical and oncologic outcomes. Ann Thorac Surg 2008;86:1632-8; discussion 1638-9. [Crossref] [PubMed]
- Schreiner W, Dudek W, Sirbu H. Is salvage surgery for recurrent non-small-cell lung cancer after definitive non-operative therapy associated with reasonable survival? Interact Cardiovasc Thorac Surg 2015;21:682-4. [Crossref] [PubMed]
- Uramoto H, Tanaka F. Salvage thoracic surgery in patients with primary lung cancer. Lung Cancer 2014;84:151-5. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. International Association for the Study of Lung Cancer staging and prognostic factors committee, advisory boards and participating institutions. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Rami-Porta R, Bolejack V, Crowley J, et al. IASLC staging and prognostic factors committee, advisory boards and participating institutions. The IASLC lung cancer staging project: proposals for the revisions of the T descriptors in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol 2015;10:990-1003.
- Chansky K, Detterbeck FC, Nicholson AG, et al. IASLC staging and prognostic factors committee, advisory boards and participating institutions. The IASLC lung cancer staging project: external validation of the revision of the TNM stage groupings in the eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2017;12:1109-21.
- Sakakura N, Mizuno T, Kuroda H, et al. The eighth TNM classification system for lung cancer: a consideration based on the degree of pleural invasion and involved neighboring structures. Lung Cancer 2018;118:134-8. [Crossref] [PubMed]
- The Japan Lung Cancer Society, General Rule for Clinical and PathologicalRecord of Lung Cancer, 8th ed., Kanehara & Co, Tokyo, Japan, 2017.
- Alexiou C, Beggs D, Rogers ML, et al. Pneumonectomy for non-small cell lung cancer: predictors of operative mortality and survival. Eur J Cardiothorac Surg 2001;20:476-80. [Crossref] [PubMed]
- Licker M, Spiliopoulos A, Frey JG, et al. Risk factors for early mortality and major complications following pneumonectomy for non-small cell carcinoma of the lung. Chest 2002;121:1890-7. [Crossref] [PubMed]
- Sakakura N, Mori S, Ishiguro F, et al. Subcategorization of resectable non-small cell lung cancer involving neighboring structures. Ann Thorac Surg 2008;86:1076-83. [Crossref] [PubMed]
- Martin J, Ginsberg RJ, Abolhoda A, et al. Morbidity and mortality after neoadjuvant therapy for lung cancer: the risks of right pneumonectomy. Ann Thorac Surg 2001;72:1149-54. [Crossref] [PubMed]
- Daly BD, Fernando HC, Ketchedjian A, et al. Pneumonectomy after high-dose radiation and concurrent chemotherapy for nonsmall cell lung cancer. Ann Thorac Surg 2006;82:227-31. [Crossref] [PubMed]
- Sonett JR, Suntharalingam M, Edelman MJ, et al. Pulmonary resection after curative intent radiotherapy (>59 Gy) and concurrent chemotherapy in non-small-cell lung cancer. Ann Thorac Surg 2004;78:1200-5. [Crossref] [PubMed]
- Pless M, Stupp R, Ris HB, et al. SAKK Lung Cancer Project Group. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. Lancet 2015;386:1049-56. [Crossref] [PubMed]
- Yang CF, Gulack BC, Gu L, et al. Adding radiation to induction chemotherapy does not improve survival of patients with operable clinical N2 non-small cell lung cancer. J Thorac Cardiovasc Surg 2015;150:1484-92. [Crossref] [PubMed]
- Shah AA, Berry MF, Tzao C, et al. Induction chemoradiation is not superior to induction chemotherapy alone in stage IIIA lung cancer. Ann Thorac Surg 2012;93:1807-12. [Crossref] [PubMed]
- Speicher PJ, Fitch ZW, Gulack BC, et al. Induction chemotherapy is not superior to a surgery-first strategy for clinical N1 non-small cell lung cancer. Ann Thorac Surg 2016;102:884-94. [Crossref] [PubMed]


