
RA-ILD: does more detailed radiological classification add something to our knowledge of this condition?
Historically, the main radiological pattern in patients with rheumatoid arthritis associated interstitial lung disease (RA-ILD) is the Usual Interstitial Pneumonia (UIP) followed by the nonspecific interstitial pneumonia (NSIP) (1). A UIP pattern at CT scan has also been associated with a worse prognosis (2,3).
Besides a UIP radiographic pattern, other recognized predictors of mortality in RA-ILD are age, male gender, extent of fibrosis on CT scan and the severity of lung function impairment (2,3). Such clinical, radiological and physiological prognostic factors are shared across other ILDs including idiopathic pulmonary fibrosis (IPF) (4).
However, recognizing a UIP pattern on a HRCT can be challenging, and the 2018 ATS/ERS/JRS/ALAT guidelines update on IPF standardized the interpretation of the radiology, with the aim to help clinicians in identifying the radiographic pattern and its prognostic implications (5).
Yamakawa et al. performed a retrospective study including 96 consecutive Japanese patients diagnosed with RA-ILD, and they classified the HRCT findings in various categories [definite UIP, probable UIP, indeterminate for UIP, NSIP, organizing pneumonia (OP), NSIP + OP, and unclassifiable according to the 2018 ATS/ERS/JRS/ALAT IPF guidelines] (6). The authors explored the possible role of such patterns as predictors of prognosis.
Noteworthy, an indeterminate pattern for UIP, was the most commonly reported (30%), followed by definite UIP (21%) and probable UIP (20%). Such prevalence is partially in contrast with data from Europe and North America where a radiographic UIP pattern was identified in the majority of cases (7,8). It is known that the distribution of reticulations and honeycombing in RA-ILD may occur in the middle or upper zones instead of being basal predominant (9). The modified HRCT classification used by the Yamakawa et al. study could have allowed this heterogeneity to be captured as indeterminate for UIP pattern. In addition, it is known that, in some cases, such radiological classification may be artificial since indeterminate and NSIP patterns on HRCT may return UIP at histology (10).
The authors did not observe prognostic differences between the different CT patterns, however radiological honeycombing was associated with a poor prognosis, likewise also recently reported by Jacob et al. (11).
These findings are of importance, also in relation to recent randomized controlled trials with anti-fibrotics: whilst the overall efficacy of nintedanib in slowing down the progression of the fibrosing process in the lung was proved in the whole cohort of patients, a sub-analysis of the results showed that pulmonary function was preserved by the drug mostly in those in the treatment arm with a classic UIP pattern (with honeycombing) compared to the controls in the placebo group (12).
Another important clinical issue, only marginally considered so far, is the occurrence of acute exacerbations (AEs) of the disease. The authors found that these acute events influenced patients’ outcome regardless of the CT pattern. The concept of AE was originally created for IPF and subsequently extrapolated to other ILDs. AE of ILD is defined as an acute worsening of dyspnea with new ground glass opacities or consolidations on chest imaging and no evidence of other causes of acute respiratory failure including pulmonary embolism, congestive heart failure, infections and pneumothorax (13). In 2016, this definition was modified to include those acute deteriorations with known etiologies, including pulmonary infections, in the context of AE-IPF. However this modification has not yet been extended to other ILDs (14). In RA-ILD the yearly incidence of AEs is between 2.58 and 11%. The risk factors for AE are similar to the prognostic factors for mortality and include advanced age, UIP pattern and performing a surgical lung biopsy. RA disease activity outside the chest does not appear to increase risk of AE (1). Mortality for AE of RA-ILD is estimated to be similar to AE-IPF (15). Considering the high mortality associated with these acute events, it is not surprising that they negatively impact disease trajectory and prognosis. An AE was the cause of death in 22% of cases in the cohort described by Yamakawa et al., particularly in patients with an UIP and NSIP/UIP pattern. Despite its fundamental role in the natural history of disease, there is still much to know about AE pathogenesis and risk factors, in particular more studies are needed to characterize AE of CTD-ILDs since the majority of literature is focused on AE of IPF.
Furthermore, health related quality of life (HrQoL) and the burden of symptoms are clinically relevant domains that are still poorly explored in CTD-ILD, although their potential prognostic value was recently highlighted. Natalini et al. showed that at baseline HrQOL in RA-ILD is worse when compared to IPF (16). After adjusting for age and pulmonary function impairment in this study, dyspnea and fatigue were predictive of poor HrQoL. A recent study found an association between overall survival and HrQoL in IPF (17). It is possible that the same associations exist in RA-ILD and other CTD-ILD, and this should be explored in future studies.
In conclusion, according to the results presented by Yamakawa et al. and to the data already reported in the literature, the presence of honeycombing and the radiological extent of the pulmonary involvement are the two variables most closely associated with a poor prognosis (Figure 1). UIP pattern seems to be associated to higher mortality in RA-ILD compared to other patterns; however not all studies are unanimous, especially after correcting for lung function impairment and type of treatment (1,3).
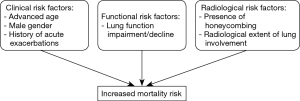
Well-designed, prospective studies including clinical and functional data such as advanced age, gender, smoking status, pulmonary function testing, history of AEs and HrQoL will be of paramount importance in understanding the trajectory and prognosis of the disease, Figure 1.
Acknowledgments
Dr. Faverio and Dr. Luppi were supported in this research by the Italian Ministry of University and Research (MIUR) - Department of Excellence project PREMIA (PREcision MedIcine Approach: bringing biomarker research to clinic).
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.111). Dr. MK reports personal fees from Boehringer-Ingelheim, grants and personal fees from Roche outside the submitted work. Dr. FL reports grants and personal fees from Roche, personal fees from Boehringer-Ingelheim, outside the submitted work. Dr. GF reports personal fees from Roche, personal fees from Boehringer Inegelheim, outside the submitted work. PF has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Juge PA, Lee JS, Ebstein E, et al. MUC5B Promoter Variant and Rheumatoid Arthritis with Interstitial Lung Disease. N Engl J Med 2018;379:2209-19. [Crossref] [PubMed]
- Assayag D, Lubin M, Lee JS, et al. Predictors of mortality in rheumatoid arthritis-related interstitial lung disease. Respirology 2014;19:493-500. [Crossref] [PubMed]
- Singh N, Varghese J, England BR, et al. Impact of the pattern of interstitial lung disease on mortality in rheumatoid arthritis: a systematic literature review and meta-analysis. Semin Arthritis Rheum 2019;49:358-65. [Crossref] [PubMed]
- Tran T, Šterclová M, Mogulkoc N, et al. The European MultiPartner IPF registry (EMPIRE): validating long-term prognostic factors in idiopathic pulmonary fibrosis. Respir Res 2020;21:11. [Crossref] [PubMed]
- Raghu G, Remy-Jardin M, Myers JL, et al. American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198:e44-68. [Crossref] [PubMed]
- Yamakawa H, Sato S, Tsumiyama E, et al. Predictive factors of mortality in rheumatoid arthritis-associated interstitial lung disease analysed by modified HRCT classification of idiopathic pulmonary fibrosis according to the 2018 ATS/ ERS/JRS/ALAT criteria. J Thorac Dis 2019;11:5247-57. [Crossref] [PubMed]
- Solomon JJ, Chung JH, Cosgrove GP, et al. Predictors of mortality in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 2016;47:588-96. [Crossref] [PubMed]
- Kelly CA, Saravanan V, Nisar M, et al. Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics – a large multicentre UK study. Rheumatology 2014;53:1676-82. [Crossref] [PubMed]
- Rajasekaran BA, Shovlin D, Lord P, et al. Interstitial lung disease in patients with rheumatoid arthritis: a comparison with cryptogenic fibrosing alveolitis. Rheumatology (Oxford) 2001;40:1022-5. [Crossref] [PubMed]
- Kim EJ, Elicker BM, Maldonado F, et al. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 2010;35:1322-8. [Crossref] [PubMed]
- Jacob J, Hirani N, van Moorsel CHM, et al. Predicting outcomes in rheumatoid arthritis related interstitial lung disease. Eur Respir J 2019;53:1800869. [Crossref] [PubMed]
- Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N Engl J Med 2019;381:1718-27. [Crossref] [PubMed]
- Luppi F, Cerri S, Taddei S, et al. Acute exacerbation of idiopathic pulmonary fibrosis: a clinical review. Intern Emerg Med 2015;10:401-11. [Crossref] [PubMed]
- Collard HR, Ryerson CJ, Corte TJ, et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am J Respir Crit Care Med 2016;194:265-75. [Crossref] [PubMed]
- Park IN, Kim DS, Shim TS, et al. Acute exacerbation of interstitial pneumonia other than idiopathic pulmonary fibrosis. Chest 2007;132:214-20. [Crossref] [PubMed]
- Natalini JG, Swigris JJ, Morisset J, et al. Understanding the determinants of health-related quality of life in rheumatoid arthritis-associated interstitial lung disease. Respir Med 2017;127:1-6. [Crossref] [PubMed]
- Furukawa T, Taniguchi H, Ando M, et al. The St. George's Respiratory Questionnaire as a prognostic factor in IPF. Respir Res 2017;18:18. [Crossref] [PubMed]


