
Airway complications after lung transplantation: benefit of a conservative bronchoscopy strategy
Introduction
Lung transplantation (LT) appeared recently in the 60s and is considered nowadays as the reference treatment of end-stage respiratory diseases (1). Complications of airway anastomosis are still “Achilles heel”, leading to morbidity and perhaps mortality, decreasing quality of life and increasing health care costs (2). The incidence of airway complications (AC) varies from 2% to 25%, depending on its definition and their symptomatic character (3-5).
Prevalence of AC is underestimated by the fact that only symptomatic patients who need endobronchial or surgical treatment are listed (6). Bronchial ischemia is the worst factor which influences the healing of the bronchial anastomosis. During LT, the systemic blood supply of the organ is not routinely restored during the transplant (7). A functional network around bronchial anastomosis is detectable only after 4 weeks (8) and vessels are present after 12–14 days (9). In consequence, pejorative aspect of anastomosis in the early postoperative period is a common way to bronchial healing, furthermore affected by several factors including donor management, high-dose steroids administration, surgical technique and perioperative management. Unfortunately, some patients do not reach a self-healed bronchial status by a spontaneous way and need intervention.
Nowadays, secondary to the lack on consensual AC grading system, transplant teams use different strategies for treatment modality and time to intervention. Many classifications are useful for clinicians to describe bronchoscopy findings and anastomotic complications after LT. The MDS Classification is the last published (10), established by a large consensus of French experts in flexible and rigid bronchoscopies and lung follow-up, using a standardized and exhaustive grading system (Table 1).
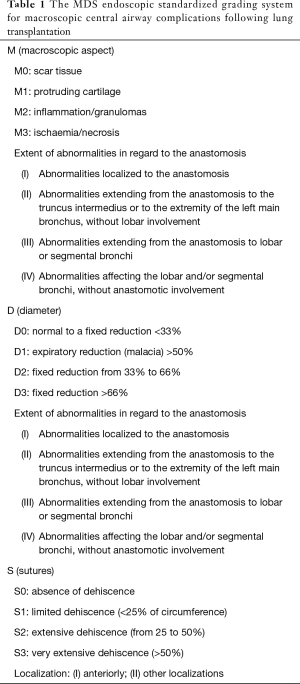
Full table
The first purpose of this study was the determination of prevalence and the characterization of risk factors for ACs. Then, we aimed to describe our active strategy of an early bronchoscopic intervention, depending on the macroscopic aspect of the bronchial anastomoses and downhill airways after LT according to the MDS classification, which determines our therapeutic attitude and clinical outcomes.
Methods
Study design
Data were reviewed from a consecutive single center series of 121 patients between August 2010 and May 2016. Combined heart-LT and redo-LTs were excluded. Single-LTs were performed through a posterolateral thoracotomy and bilateral single LT by a bilateral trans-sternal anterior thoracotomy (clamshell incision). Dissection avoided injury of bronchial arteries of the recipient. All lungs were from donors after brain death, harvested by the same surgeon. Before antegrade flush, prostaglandin E1 (Prostin VR, Upjohn, Puurs, Belgium) was injected into the pulmonary artery. Lungs were flushed in an anterograde fashion with 5 Liters of Celsior® (Genzyme, Catalent Limoges S.A.S., France). Before implantation, a 1 L complementary retrograde flush via pulmonary veins was performed on donor’s lung. Conventional anastomotic technique was performed by trimming the mainstem donor bronchus to within 1 or 2 rings of the secondary carina and then using a running suture for the membranous wall and separated stiches for the cartilaginous part (4-0 Prolene™, polypropylene, Surgi-pro, US Surgical, Norwalk CT or PDS™ II, polydioxanone, Ethicon Inc., Somerville, NJ, USA) to do an end-to-end anastomosis. Intentional telescoping is not performed except when there is a size difference, then the smaller bronchus was allowed intussuscept into the larger airway. Peribronchial fatty tissues of the donor were patched around with resorbable stiches whenever possible (Vicryl™, Ethicon, Somerville, NJ, USA). Since 2013, all the patients of our Lung Transplant program have systematic and repeated amphotericin nebulization to avoid Aspergillus colonization. All patients underwent routine flexible bronchoscopy at the end of lung anastomosis during surgery and every two days during the first 15 days after transplantation, then once time a week to reach one month of follow up. Further bronchoscopies were performed every 2 months until the end of the year, or in urgent necessity (symptoms or forced expiratory volume in one second reduction during follow up). The immunosuppressive protocol remains unchanged during the study period:induction therapy with basiliximab and bolus of methylprednisolone in the operative room, followed by tacrolimus, mycophenolate mofetyl and steroids.
Data
We did a retrospective analysis of all medical and PMSI data to search for airway anastomotic complications (AC). We gathered recipient variables of pre- and perioperative period associated with the occurrence of AC.
Variables with biological plausibility, significance in univariate analysis and supporting literature with occurrence of AC were included in the multivariate model (Table 2).

Full table
The statistical unit is the patient, for “patient-level” data in uni and multivariate models. The statistical unit is the bronchial anastomosis, when we studied factors related to each anastomosis. A SLT matched with 1 unit and BSLT with 2 units for each patient.
Statistical analysis
Qualitative variables were expressed as count with percentage, and continuous variables as mean and standard deviation, unless otherwise stated. Mean and median values were displayed with standard deviation and range values, respectively. Odds ratios (OR) were presented with their 95% confidence intervals (CI). Statistical significance of continuous data was calculated using the unpaired two-tailed t-test for normally distribution. Variables with biological plausibility, proven literature support in the occurrence of AC were studied by a univariate analysis with the chi square or Fisher’s exact test. Variables with significance were included in a multivariate model with a stepwise logistic regression, with P≤0.2 used as a cut-off for inclusion. Model discrimination was assessed by the c-index, which is identical to the area under the receiver operating characteristic curve. All reported P values are two-sided and P<0.05 was considered statistically significant. Overall survival rates were estimated with the Kaplan-Meier method and compared with the log-rank test. Statistical calculations were performed with SAS software, version 9.3 (SAS institute, Inc., Cary, NC, USA).
Results
Overall analysis
A total of 121 LT, 39 single-lung (SLT) and 82 bilateral sequential lung transplantation (BSLT) were performed for a total of 203 bronchial anastomoses. Two patients died until the first postoperative day (POD), one from a large vena cava dehiscence (during the removal of inferior venous cannula, inserted percutaneously in the femoral vein) and the second from a major primary graft dysfunction (PGD) and were excluded. Gender distribution was 70 males (58%) and 51 females (42%), with a mean age of 42 years (8–65 years). The indications for LT were cystic fibrosis (n=56), emphysema (n=30), pulmonary fibrosis (n=23), and others causes (n=12) (Table 2).
AC concerned 41 anastomoses in 28 patients (prevalence: 23%), and all occurred during the first postoperative year with a median time of 73 days (range 10–198 days) from transplantation to AC diagnosis. The distribution of AC according to the MDS classification is presented in Table 3. Gender distribution was 16 males (57%) and 12 females (43%). All AC were treated by at least one intervention of rigid bronchoscopy. Both sides were indifferently concerned by AC (21 right anastomoses, 20 left).
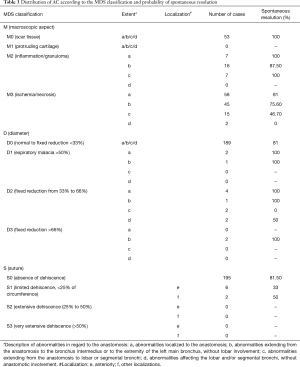
Full table
Multivariate analysis of predictive factors of AC
At a “patient-level”, the post-operative infection by Aspergillus species (OR 2.7, 95% CI: 1.08–6.75; P=0.033) and increasing Body Mass Index (OR 1.12, 95% CI: 1.02–1.25; P=0.025) were withheld as predictive factors for AC. At the “anastomosis-level”, emphysema (OR 2.4, 95% CI: 1.02–5.6; P=0.045), post-operative infection by Aspergillus (OR 2.3, 95% CI: 1–5.1; P=0.05), premature dehiscence until post-operative day (POD) 20 (OR 11.2, 95% CI: 1.7–76; P=0.01), latest or persistent dehiscence until POD 73 (OR 11.5, 95% CI: 6–67; P=0.007) and cold ischemia >264 min (OR 2.45, 95% CI: 1.08–5.6; P=0.03) were associated with ACs.
Survival analysis and long-term outcome
There was no statistically significant difference in overall survival between patients with AC and the control group [hazard ratio (HR) 1.6, 95% CI: 0.6–4.6; P=0.22]. One- and 2-year survivals for the AC group were respectively 96.5% and 85%, and for the control group, 92% and 81% (Figure 1). Median term of follow-up was 876 days.
Treatment characteristics and outcome
At POD 20 after transplantation in mean, 59% bronchial anastomosis presented a macroscopic aspect of ischemia or necrosis (M3 stage, n=120) and 7% (n=14) with a diameter complication. For MDS stages from M0 to M3a, the probability of spontaneous resolution was 93.7% compared with 61.1% for stages M3b and higher (Table 2). At POD 73, 141 anastomoses (70%) were in complete healing (M0 stage). Among the 49 anastomoses which presented necrosis or ischemia (M3 stage), 41 required rigid bronchoscopy. Anastomoses diameter was normal in 167 (82%). Between POD 20 and 73, 4 patients presented a spontaneous dehiscence resolution, 4 had a persistent one (S1) and 3 patients developed a later dehiscence of S1 type.
An average of 3 bronchoscopy procedures per patient was realized, at a mean time of 103±48 days from transplant. The median length of stay in the bronchoscopy unit was 2±1 days.
During the first intervention, after a careful airway intubation and an exhaustive assessment of bronchus status, a mechanical debridement with a rotary forward motion of the hollow metal tube was realized, or with cup forceps was performed for 95% of the anastomoses. Dilatation was performed with tubes of increasing diameter for 73% of AC. There was no fibrotic stenosis at this early post-operative course. In presence of bronchial dehiscence, surgical clips and/or polypropylene sutures were systematically encountered and removed (n=7, classed S1).
At the end of the bronchial clearance, malacia defined by expiratory diameter collapse of >50%, concerned 9 AC and 7 required a stent implantation through the hollow tube, with a slight over-sizing of 20%. Three Silicone Dumon stents and 4 fully covered self-expandable metallic stents (Fc-SEMS) were inserted (Silmet®, Novatech, La Ciotat, France).
A second bronchoscopic procedure was required for 20 patients (27 anastomoses) at POD 185±105 (range 97–463). Anastomoses needed more dilatation (93%, n=25) than debridement (81%, n=22). Usually, AC classed D3 became D2 when the underlying bronchial frame was soft, or became normal (D0) when occlusion was made of aspergillus necrosis. However, all the AC with malacia before dilatation had a persistent collapse after. Thirteen stents were implanted in 12 AC (7 Fc-SEMS for fixed reduction, 6 silicone stents for bronchomalacia).
The following procedure concerned 9 or less patients, for fibrotic stenosis or malacia. Less debridement but more dilatations were required. At one-year of follow-up, 25% of AC still evolved on a necrotic fashion while 25% were severely fixed by a fibrotic stenosis. The remaining AC needed stents for malacia (6 Fc-SEMS and 2 silicone stent).
AC was considered as definitely treated for 16 patients with AC (28 anastomoses, median follow-up of 820 days (range, 251–2,052 days). Nine patients are still under stenting or evolving AC (11 anastomoses, median of last follow-up 162 days).
Stent complications
All the 40 stents (20 fully covered SEMS and 20 silicone stents) were used in the main bronchus and 1 Y silicon stent was fit on the main carina. Stent deployment was successful in all patients without procedure-related events, or major complication during follow-up. There were minor complications such as tenacious secretions (6 Fc-SEMS, 9 silicone stents), moderate granulomas (10 Fc-SEMS, 11 silicone stents). There were 8 asymptomatic stent migrations with both types of stents. Superior or middle lobar occlusions couldn’t be avoided with Fc-SEMS in 5 cases but were avoided with windowed silicone stents. Median follow-up after the last intervention was 313 days for the AC group. Stents stayed 235±218 days (range, 4–1,078 days, median 175 days), and 12 stents (7 in silicone, 5 Fc-SEMS) are still in place, since 243±150 days.
Discussion
The current study, including 121 LTs in a 6-year period, shows a significant AC prevalence of 23%. Most published series usually described a range of 2% to 25% (3-5) of AC, with a disparity explained by underreporting. Since 2010, we conduct a prospective program of early mechanic debridement of anastomoses, under rigid bronchoscopy, to prevent ACs. We highlight that early dehiscence, emphysema, post-operative infection with Aspergillus species (before day 90) and cold ischemia time are risk factors of AC. In COPD patients, peribronchial tissues and vascular network are scarce compared with cystic fibrosis or idiopathic pulmonary fibrosis patients. In consequence, the relative ischemia after the LT may be higher for COPD who had already chronic ischemia, and may explain its importance in AC occurrence. Previous reports hypothesized that once the mucosal barrier is breached by local ischemia, contaminated environment increased local ischemia (11). In our series, Aspergillus was the main fungal specie which seems to hamper the bronchial healing. Concerning the immunosuppressive regimen, we employ the same as the “treatment 3” used from 2009 to 2012 by the Lung transplantation Group of Strasbourg, France, who founded a rate of bronchial complications of 25% (5).
In our study, AC appears to be frequent but less severe in term of dehiscence, and with no preferential lateralization. Our results may be explained by recent works on animal ex vivo models, showing that Celsior® decreased airway resistance and increased gas exchange performance, compared to Perfadex® (12). The Celsior® solution contains antioxidant substances and further studies are required to understand the underlying mechanisms of both solutions and confirm the superiority of one on another (13). In clinical lung TP, celsior didn’t showed a strong improvement of clinical outcomes or survival against the gold standard low-potassium dextran solution (LPD, Perfadex). Despite unfavorable ischemic time in the Celsior group, a large retrospective study of Hannover’s team found a tendency of PGD resolving faster and a significant improvement of Bronchiolitis obliterans syndrome-free survival, compared with LPD (14). In AC with dehiscence, surgical clips or terminal nodes were systematically founded and necrotic aspect was indifferently observed with resorbable or polypropylene stitches. As these materials appeared at different times of AC evolution, it is impossible to know their causal relationship with dehiscence. Moreover, individual stitches on cartilaginous part may have limit dehiscence in this fragile area. Other teams with lower rate of AC no longer use non-resorbable monofilament sutures and individual stitches, but changed in the same time many surgical procedural aspects. It could be a way forward to decrease our rate of limited dehiscence.
Concerning the early post-operative course (POD 20), we counted 120 necrotic anastomoses (M3) in 89 patients, a prevalence of 60%. In our experience, 60% of these anastomoses evolved favorably and self-healed without any therapeutic intervention. Instead of Leuven team, we didn’t consider these airway abnormalities as AC, because airway anastomoses remain profoundly ischemic for some weeks after transplantation before complete and spontaneous healing (15,16). Anyway, these lesions resulted in our experience and in literature in increased endoscopic monitoring, with a conservative and wait-and-see attitude (10).
There was no statistically significant difference in overall survival between the AC group treated conservatively and the controls. The bronchoscopic interventions helped us to manage conservatively the AC group on a 6-year period, neither without major complications nor without graft removal. Mean length of hospital stay was 2 days and neither costly device such as lasers, microdebriders or dilatation balloons was useful. Our patients welcomed the gain of such short and effective procedures, under general anesthesia. In our center, we consider that a well-trained team in rigid bronchoscopy may efficiently treat various type of AC, with an exhaustive action on macroscopic, diameter and dehiscence complications and without costly instruments. Last but not least, this technique improves the accuracy of local damage assessment in such a dynamic environment, with a more precise sizing before bronchial stenting when necessary.
The MDS classification employed in our experience is less exhaustive than others previous described, at the early stage, but keep it simpler. This standardized and exhaustive classification helps physicians to assess the severity rate of AC for the better therapeutic option, to restore safely and rapidly better bronchial conditions to improve graft oxygenation. A description of an isolated M1 type (malacia), without diameter repercussion, generally doesn’t require any intervention. A M2 injury (granulomas, inflammation) requires a debridement intervention when associated with a significant reduction of caliber (more than 33%, ≥ D2). In case of necrotic, fungal or ischemic injuries, local toilet under flexible bronchoscopy is firstly indicated, if the area concerned is small (M3a) or with no significant diameter reduction (<33%). An isolated reduction of caliber is not an independent factor for clinical symptoms. It is not in itself the only factor for therapeutic decision-making, except for major expiratory malacia (D1, >50%) alone in a single-lung situation. Concerning the suture, dehiscence of the anterior wall has more pejorative prognosis significance than a lateral or a posterior one, due to the higher risk of vascular fistulae. We experienced only limited dehiscence of the lateral wall, which may explain the absence of surgical treatment.
Novel fully-covered SEMS stents were widely implanted thanks to their great ease to use. In the literature, Fc-SEMS suffer from misusing and are criticized for high short-term complications (75% of migration, 60% of stent fractures, before 12 weeks!). In these studies, 82% of these cylindrical stents were implanted for tracheal stenosis (17). When correctly sized in bronchial benign strictures following LT, Fc-SEMS and silicone stents are efficient, according to our experience (18-21). On the contrary, reports and notifications of Food and drug Administration alert physicians to serious complications associated with non-covered metallic stents, in benign airway disorders, since 20 years (2,22,23).
This is a single-institution study, representing the results of a fixed team of surgeons across time, but with variations in techniques and increase in the level of experience. Subtle differences among surgeons are undocumented and cannot be take in account, despite the repetitive operative technique employed. However, our good results of conservative management of AC must be weighed against the relative small sample size of our series. Finally, the high occurrence of AC highlights the fact that peri-operative care and transplant technique can still be improved in our center.
In conclusion, our transplant experience is in accordance with the literature in term of survival results and predictive factors of ACs. The early care of ACs under rigid bronchoscopy seems to contain their evolution, by a conservative way, respecting the scalable process of bronchial healing. In our knowledge, it is the first report of consecutive transplanted patients with no surgical treatment for AC. Furthermore, it is the first series using widely and safely the third generation of fully covered metallic stents, adapted to situations of tight strictures or tortuous airways. According to intra-operative findings, the gold standard silicone stents still have an important place in case of malacia or for long term airway supporting. Bronchial healing is still an important challenge after LT and rigid bronchoscopy has a key place in the therapeutic strategy, as a multifunction and minimally invasive tool.
Acknowledgments
The authors acknowledge the enormous contribution of the nurses, doctors and other people who make possible our Lung Transplantation program every day, for many years.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.21). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. In compliance with the request from the CNIL (article 34 of the French “Informatique et Libertés” [Information Technologies and Freedom] law No. 78-17 dated 6 January 1978 and No. 2004-801 dated 6 August 2004), this study is the subject of a declaration filed with the French “Commission Nationale de l’Informatique et des Libertés” (National Commission on Information Technologies and Freedom). The Ethics committee of the University of Lyon-Saint Etienne, France, approved for the collection of patient data and analysis within the setting of a retrospective study design.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stehlik J, Hosenpud JD, Edwards LB, et al. ISHLT International Registry for Heart and Lung Transplantation--into the fourth decade, from strength to strength. J Heart Lung Transplant 2013;32:941-50. [Crossref] [PubMed]
- Murthy SC, Blackstone EH, Gildea TR, et al. Impact of anastomotic airway complications after lung transplantation. Ann Thorac Surg 2007;84:401-9, 409-4.
- Awori Hayanga JW, Aboagye JK, Shigemura N, et al. Airway complications after lung transplantation: Contemporary survival and outcomes. J Heart Lung Transplant 2016;35:1206-11. [Crossref] [PubMed]
- Weder W, Inci I, Korom S, et al. Airway complications after lung transplantation: risk factors, prevention and outcome. Eur J Cardiothorac Surg 2009;35:293-8; discussion 298. [Crossref] [PubMed]
- Olland A, Reeb J, Puyraveau M, et al. Bronchial complications after lung transplantation are associated with primary lung graft dysfunction and surgical technique. J Heart Lung Transplant 2017;36:157-65. [Crossref] [PubMed]
- Herrera JM, McNeil KD, Higgins RS, et al. Airway complications after lung transplantation: treatment and long-term outcome. Ann Thorac Surg 2001;71:989-93; discussion 994. [Crossref] [PubMed]
- Shennib H, Massard G. Airway complications in lung transplantation. Ann Thorac Surg 1994;57:506-11. [Crossref] [PubMed]
- Pearson FG, Goldberg M, Stone RM, et al. Bronchial arterial circulation restored after reimplantation of canine lung. Can J Surg 1970;13:243-50. [PubMed]
- Siegelman SS, Hagstrom JW, Koerner SK, et al. Restoration of bronchial artery circulation after canine lung allotransplantation. J Thorac Cardiovasc Surg 1977;73:792-5. [Crossref] [PubMed]
- Dutau H, Vandemoortele T, Laroumagne S, et al. A new endoscopic standardized grading system for macroscopic central airway complications following lung transplantation: the MDS classification. Eur J Cardiothorac Surg 2014;45:e33-8. [Crossref] [PubMed]
- Yserbyt J, Dooms C, Vos R, et al. Anastomotic airway complications after lung transplantation: risk factors, treatment modalities and outcome-a single-centre experience. Eur J Cardiothorac Surg 2016;49:e1-8. [Crossref] [PubMed]
- Menezes AQ, Pêgo-Fernandes PM, Cardoso PFG, et al. Comparison of Celsior and Perfadex lung preservation solutions in rat lungs subjected to 6 and 12 hours of ischemia using an ex-vivo lung perfusion system. Clinics 2012;67:1309-14. [Crossref] [PubMed]
- Xiong L, Legagneux J, Wassef M, et al. Protective effects of Celsior in lung transplantation. J Heart Lung Transplant 1999;18:320-7. [Crossref] [PubMed]
- Gohrbandt B, Simon AR, Warnecke G, et al. Lung Preservation With Perfadex or Celsior in Clinical Transplantation: A Retrospective Single-Center Analysis of Outcomes. Transplantation 2015;99:1933-9. [Crossref] [PubMed]
- Gade J, Qvortrup K, Andersen CB, et al. Bronchial transsection and reanastomosis in pigs with and without bronchial arterial circulation. Ann Thorac Surg 2001;71:332-6. [Crossref] [PubMed]
- Dhillon GS, Zamora MR, Roos JE, et al. Lung transplant airway hypoxia: a diathesis to fibrosis? Am J Respir Crit Care Med 2010;182:230-6. [Crossref] [PubMed]
- Dooms C, De Keukeleire T, Janssens A, et al. Performance of fully covered self-expanding metallic stents in benign airway strictures. Respiration 2009;77:420-6. [Crossref] [PubMed]
- Kim H. Stenting therapy for stenosing airway disease. Respirology 1998;3:221-8. [Crossref] [PubMed]
- Bolliger CT. Prosthesis: indications, contraindications and follow-up. Rev Mal Respir 1999;16:665-72. [PubMed]
- Van De Wauwer C, Van Raemdonck D, Verleden GM, et al. Risk factors for airway complications within the first year after lung transplantation. Eur J Cardiothorac Surg 2007;31:703-10. [Crossref] [PubMed]
- Dutau H, Cavailles A, Sakr L, et al. A retrospective study of silicone stent placement for management of anastomotic airway complications in lung transplant recipients: short- and long-term outcomes. J Heart Lung Transplant 2010;29:658-64. [Crossref] [PubMed]
- Health C for D and R. Public Health Notifications (Medical Devices) - FDA Public Health Notification: Complications from Metallic Tracheal Stents in Patients with Benign Airway Disorders. [cité 9 oct 2016]. Available online: http://www.jsre.org/info/0801_fda.pdf
- Gottlieb J, Fuehner T, Dierich M, et al. Are metallic stents really safe? A long-term analysis in lung transplant recipients. Eur Respir J 2009;34:1417-22. [Crossref] [PubMed]



