
The tricuspid valve in review: anatomy, pathophysiology and echocardiographic assessment with focus on functional tricuspid regurgitation
Introduction
Tricuspid valve (TV) disease, mostly seen as tricuspid regurgitation (TR) in adults, is the most common right-sided valvular heart lesion. Physiological trace to mild TR is very common and anatomically normal (1,2). For decades, even moderate or greater TR has been considered a benign condition and therefore has been under-treated (3). Most recently, moderate and severe TR have been established as independent predictors of mortality in patients with significant left-sided valve disease and LV dysfunction (4-6). Clinically significant TR, defined as moderate or greater, is more common in women and its prevalence increases by age (7). In a community study performed in Olmsted County, Minnesota, overall TR prevalence was 0.55%, 0.47% in men and 0.59% in women (7). This translates into approximately 1.6 million US residents. As the prevalence of TR increases with age, the burden of TR is expected to increase in the context of a globally aging population.
Primary (organic) TR can be inherited or acquired, and accounts for 10% of cases of TR in adults (8). Inherited causes include Ebstein’s anomaly, atrioventricular defects, and myxomatous prolapse. Acquired primary TR can be caused by rheumatic disease, carcinoid syndrome, infective endocarditis, cardiac implantable electronic device leads, trauma (for example, from repeated endomyocardial biopsies after cardiac transplantation, or from blunt trauma with papillary muscle rupture), drugs, and endomyocardial fibrosis.
Functional (secondary) TR constitutes approximately 90% of TR and is due to a heterogenous group of etiologies that cause right atrial (RA) or right ventricular (RV) dilation. This results in annular dilation and tethering of the TV leaflets (9). Common causes of RV/RA dilation include pulmonary hypertension (PH), longstanding atrial fibrillation, left-sided heart failure, mitral regurgitation or stenosis (MR or MS), and significant left-to-right shunting.
The clinical impact of isolated TR has been demonstrated in multiple studies. In an earlier study by Nath et al., within a cohort of 5,223 patients, the presence of severe TR was associated with decreased survival after adjusting for PH and ejection fraction (10). A study from Olmstead County, Minnesota demonstrated that mortality in patients with greater than or equal to moderate isolated TR was higher than in the matched cases with trivial TR (P=0.0014; matching for age, sex, atrial fibrillation, ejection fraction, comorbidity index) (7). In a retrospective analysis by Chorin et al. (11), hazard ratio for overall mortality was 1.15 for moderate TR [95% confidence interval (CI), 1.02–1.30, P=0.024] and 1.43 for severe TR (HR 1.43; 95% CI, 1.08–1.88, P=0.011) when compared to no or minimal TR.
Despite the negative impact on outcomes, based on the current AHA/ACC guidelines (12), the only class I indication for TV repair or replacement for severe functional TR is at the time of left-sided valve surgery. Surgical therapy for patients with less than severe functional TR with tricuspid annular dilation, defined as tricuspid annular dimension >40 mm on TTE (>21 mm/m2 when indexed to body surface area) or >70 mm on direct intraoperative measurement, or prior evidence of right heart failure at the time of left-sided valve surgery is controversial. High-quality randomized controlled trials and current-era observational studies examining management of isolated TR are lacking. Valve replacement is rarely performed in this setting and clinical practice guideline recommendations are based on expert opinion and limited data. Isolated TV repair has a class IIb indication—owing to the historically poor surgical outcomes, with a reported mortality of 8–10% (13), in the context of delayed referral, advanced heart failure, RV dysfunction, and frequent concomitant cardiac cirrhosis.
With the aging population, increased recognition of the impact of functional TR on outcomes, and novel therapeutic avenues, the interest in TV disease has been rekindled. The advent of transcatheter interventional approaches, which started with left-sided valve disease, has begun shifting towards the TV with several new percutaneous therapies under development. These procedures and the associated clinical trials necessitate a thorough understanding of the TV anatomy, pathological changes, quantification of the severity of the disease, and echocardiographic imaging of the valve.
TV anatomy (Figure 1)
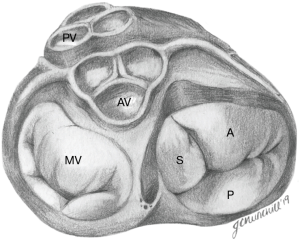
The normal TV complex includes a fibrous annulus, usually three leaflets (anterior, septal, posterior), chordae tendinae, papillary muscles, RA myocardium, and RV myocardium. One study by Lama et al., which evaluated 36 adult formalin-fixed human hearts, showed that the number of TV leaflets can vary from three to as many as seven, and accessory leaflets are often the smallest in size (14).
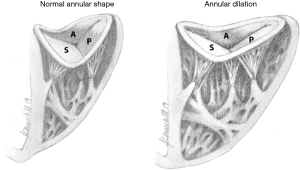
The TV is the largest of the four cardiac valves and its area is between 7 and 9 cm2. The tricuspid annulus (TA) is an asymmetrical, saddle-shaped ellipsoid that is dynamic in nature, allowing it to change with varying loading conditions. Its most superior points are the anteroseptal portion (near the RV outflow tract and aortic valve) and the posterolateral portion. Its most apical point is the posteroseptal portion (near the inflow of the coronary sinus) and the anterolateral segment (Figure 2). In normal subjects, the TA circumference is 12±1 cm and the area is 11±2 cm2 (15,16). TA dimensions increase during atrial systole and again in late ventricular systole/early diastole by about 30% (17). Leaflets are usually semicircular or triangular in shape and are attached basally to the fibrous annulus. The distal quarter to third of the leaflets receives insertions of chordae tendineae. The anterior (or superior) leaflet is usually the largest and most mobile, abutting the outflow of the RV. The posterior and septal leaflets are more variable in size and mobility, with the septal leaflet being the least mobile. In the absence of a congenital abnormality (i.e., Ebstein’s anomaly), the septal leaflet inserts into the septum ≤10 mm apical to the septal insertion point of the anterior mitral leaflet, rendering it more apically displaced than the mitral valve. The posterior tricuspid leaflet often has multiple scallops; however, in some, a clear division between the anterior and posterior leaflets is not always apparent (18).
The TV has three main papillary muscles, and numerous small ones which typically connect to the septal and posterior leaflets. The anterior papillary muscle is often the largest, arises from the moderator band, and is located at the commissure between anterior and posterior leaflets. Chordae tendineae are fibrous strands that attach valve leaflets to papillary muscles—or, unlike the mitral valve, directly to the myocardial wall of the RV. Primary chordae are attached to the free edge of the leaflets and are most important in preventing regurgitation. Secondary chordae, on the other hand, insert into the basal portion of the ventricular surface of the leaflet.
TV physiology and mechanisms of functional TR
The TA is less stiff and slightly larger than the mitral valve annulus, and therefore is more likely to dilate with ventricular enlargement. It becomes planar and circular as the RV free wall becomes displaced, stretching the anterior and posterior portions of the TA. The septal portion of the TA remains relatively fixed, less affected by RV dilatation (19) (Figure 2). TR is highly dependent on annular dilation and on animal models, significant TR occurs with only 40% dilatation (20) compared to 75% dilatation for mitral regurgitation (17).
As mentioned earlier, the causes of functional (secondary) TR (FTR) are typically related to RV or RA dilation. Originally, excessive RV afterload due to PH was thought to be the sole cause of FTR, and therefore it has been the main focus of the current guidelines on management of valvular heart disease (12). More recently, epidemiological studies point to the high prevalence of FTR in the absence of PH. A new entity, idiopathic FTR, has been described, where significant TR is identified without an overt cause (LV dysfunction, pacemaker lead impingement, primary TR) or any other significant concomitant valve disease (21). In one study, idiopathic FTR represented 12% of patients with TR and showed a strong link to advanced age and atrial fibrillation. In the same study, evaluating the mechanisms of TR, the authors showed that idiopathic FTR was associated with marked annular enlargement without significant valvular tethering, whereas in PH related FTR there was valvular tethering with tenting above the annular level leading to reduced coaptation and moderate annular enlargement.
The role of ventricular-valvular interaction was also apparent, where PH-related FTR and RV remodeling led to a larger, longer, and more elliptical ventricle with more valve tenting and a larger effective orifice area. Conversely, the basal RV was larger and exhibited conical deformation in patients with idiopathic FTR.
Imaging the TV
Transthoracic echocardiography (TTE)
Two-dimensional (2D) TTE imaging of the TV can be challenging due to its complex geometry. Three-dimensional (3D) TTE acquisitions of datasets including the TV can be performed from the best 2D acoustic window to help in visualization of all the TV leaflets en-face (22). Comprehensive assessment of the TV from multiple windows is essential for accurate diagnosis and quantification of TV disease. The most recent American Society of Echocardiography (ASE) guidelines outline the recommended views for performing a comprehensive evaluation of the TV, however due to anatomic variability of the TV and the varying degrees of transducer angulation, identification of each TV leaflet on standard transthoracic views remains controversial.
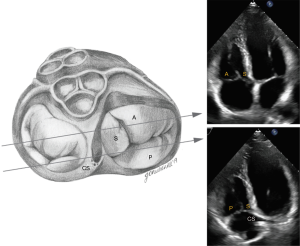
Using multiple windows and understanding the anatomy of TV and surrounding structures should help with accurate assessment of the leaflets. Based on the ASE Guideline document (23) on right heart imaging, in the short-axis (SAX) view at the aortic level, the tricuspid leaflet adjacent to the aortic valve is the septal leaflet and the leaflet attached to the basal anterior RV wall is the anterior leaflet. However, based on 2D interrogation of the TV using knowledge derived from 3-dimensional echocardiography, Addetia et al. showed that various combinations of valve leaflets could be visualized (anterior-posterior in 41%, posterior-septal in 23%, anterior-septal in 11%, anterior alone in 13%, all three in 12%) (24). Due to the apical position of the septal leaflet, with extreme anterior angulation only the anterior leaflet may be seen. As the lateral portion of the annulus dilates and loses its saddle shape, the anterior and posterior leaflets may be imaged, and as the transduced is angled toward the left ventricular outflow tract, the septal and posterior leaflets may be seen (Figure 3).
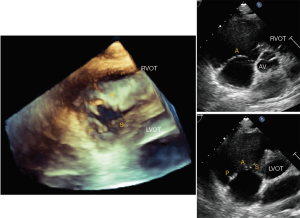
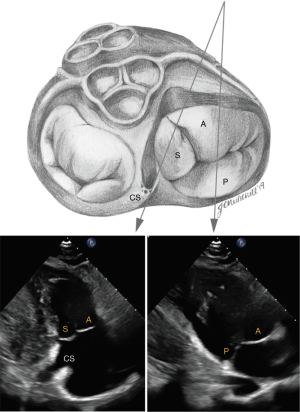
In the parasternal RV inflow view, the anterior leaflet is always visualized closest to the ultrasound beam, and based on the angulation of the transducer, either the posterior or septal leaflets can be seen. The commissure between the posterior and septal leaflet is usually located close to the coronary sinus orifice. If the coronary sinus orifice and the interventricular septum are in view, the septal leaflet is more likely to be imaged; otherwise, when the transducer is angled more acutely inferiorly and to the right, posterior leaflet is more likely to be in view (Figure 4). In apical 4 chamber view, the septal leaflet is always seen attached to the septum, but the opposing leaflet could be either the anterior leaflet (when the transducer is angulated anteriorly and the LVOT is in view) or the posterior leaflet (when posterior angulation brings the coronary sinus into view) (Figure 5). In the RV focused view, the posterior and septal leaflets are almost exclusively seen (24).
Transesophageal echocardiography (TEE)
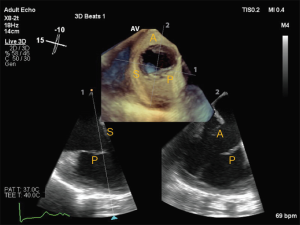
Complete assessment of the TV valve by TEE is outlined in the ASE Guidelines for performing a comprehensive TEE examination (25). The standard mid-esophageal (ME) 4 chamber view is often the first image obtained and septal and anterior leaflets are typically imaged. Simultaneous orthogonal biplane imaging across the non-septal leaflet may aid in confirming the anterior leaflet, as this leaflet is typically seen adjacent to the aorta. Images obtained at the gastroesophageal junction are ideal for comprehensive evaluation of the TV and for acquiring 3D volumes, as the left-sided structures are often out of view (Figure 6). Slowly increasing the omni-plane from 0o to 130–140o will show each of the TV leaflets. Transgastric views are especially helpful in simultaneous visualization of all the TV leaflets and is commonly used for planning of transcatheter procedures. The ME inflow-outflow view (at 50–70o) as well as the ME modified bicaval view are useful for color flow Doppler and spectral Doppler, as TR is often directed toward the interatrial septum and thus well-aligned with the insonation beam in these views. Finally, 3D TEE significantly improves the accuracy of imaging, as it has the advantage of simultaneously visualizing all leaflets and associated structures. ASE guidelines suggest obtaining a view of the TV from either the 0o to 30o ME-4 chamber view, tilted so that the valve is centered in the imaging plane, or the 40o transgastric view with anteflexion. It is essential that the acquisition volume encompasses the surrounding anatomical landmarks to help recognize the individual TV leaflets. By convention, en-face view of the TV displays the septal leaflet at the 6 o’clock position, with aorta to the left of the screen.
Quantification of TR
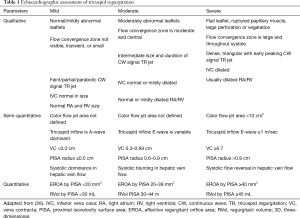
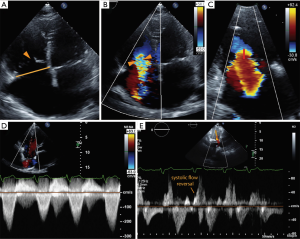
A detailed integrated approach to grading TR severity is emphasized in the ASE (26) and European Association of Echocardiography guidelines (27). Table 1 shows qualitative and quantitative parameters of severe TR based on 2D measurements (Figure 7). More recently, 3D parameters have gained interest due to the limitations of 2D parameters. Quantification of TR using 2D PISA-derived effective regurgitant orifice area (EROA) has recently been validated and is relatively easy to perform (28,29); however, it is subject to all of the limitations of its application in mitral regurgitation and can underestimate TR, owing to contour flattening and the more complex and noncircular shape of the TA (30). To overcome this, 3D echocardiography provides an accurate cross-sectional area of the vena contracta (3D VCA) and may improve the understanding of both the mechanism and severity of TR, assisting in clinical decision-making regarding disease management (30,31). A few studies have shown the utility of 3D VCA to quantify TR, most of them using 2D parameters to reference the standard (32-34). One recent study by Utsunomiya et al. used regurgitant volume derived from 3D datasets of the entire RV, with validation from cardiac magnetic resonance (31). Velayudhan et al. (32) showed 3D TTE planimetered VCA of >0.75 cm2 was the most sensitive cutoff (sensitivity, 85.2%; specificity 82.1%), whereas Song et al. (34) demonstrated an optimal cutoff of 0.57 cm2 for severe TR. Similarly, in a study by Chen et al. (33), receiver operator characteristic curve showed that the best cutoff for 3D VCA was 0.36 cm2, with a sensitivity of 89% and specificity of 84% in diagnosing severe TR. The study also found that 3D VCA correlated with other quantitative and semi-quantitative parameters in those with primary TR, a regular rhythm, and a circular orifice, but was not reliable in atrial fibrillation. Finally, Utsunomiya et al. demonstrated that three-dimensional VCA at an optimal cutoff value of 0.61 cm2 yielded a sensitivity of 78% and specificity of 97% to differentiate moderate from severe TR. The best cutoff values in the subgroup with primary TR and functional TR were 0.63 and 0.51 cm2, respectively (31). Furthermore, suggestions have been made towards an expanded TR grading scheme to account for “massive” TR (EROA 60–79 mm2) and “torrential” TR (EROA ≥79 mm2) that currently all fall within the same “severe” TR grade (35). The rationale for this expanded grading scheme comes from the experience in early feasibility trials for percutaneous tricuspid interventions in which patients with “torrential” TR showed significant TR improvement after treatment that could not be captured within the current grading scheme.
Full table
Conclusions
The normal function of the TV is an interplay between a complex anatomical structure, RV function, and loading conditions. An understanding of normal tricuspid anatomy and physiology is crucial in comprehending pathology and in successful echocardiographic assessment of functional TR. These considerations are vital in an era of increasing transcatheter tricuspid interventions and a greater appreciation for the consequences of TR.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Christos G. Mihos) for the series “Novel Concepts in Cardiopulmonary and Structural Heart Disease” published in Journal of Thoracic Disease. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.02.42). The series “Novel Concepts in Cardiopulmonary and Structural Heart Disease” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hung J. The pathogenesis of functional tricuspid regurgitation. Semin Thorac Cardiovasc Surg 2010;22:76-8. [Crossref] [PubMed]
- Badano LP, Muraru D, Enriquez-Sarano M. Assessment of functional tricuspid regurgitation. Eur Heart J 2013;34:1875-85. [Crossref] [PubMed]
- Friedman G, Kronzon I, Nobile J, et al. Echocardiographic findings after tricuspid valvectomy. Chest 1985;87:668-70. [Crossref] [PubMed]
- Koelling TM, Aaronson KD, Cody RJ, et al. Prognostic significance of mitral regurgitation and tricuspid regurgitation in patients with left ventricular systolic dysfunction. Am Heart J 2002;144:524-9. [Crossref] [PubMed]
- Hung J, Koelling T, Semigran MJ, et al. Usefulness of echocardiographic determined tricuspid regurgitation in predicting event-free survival in severe heart failure secondary to idiopathic-dilated cardiomyopathy or to ischemic cardiomyopathy. Am J Cardiol 1998;82:1301-3, A10.
- Benfari G, Antoine C, Miller WL, et al. Excess Mortality Associated With Functional Tricuspid Regurgitation Complicating Heart Failure With Reduced Ejection Fraction. Circulation 2019;140:196-206. [Crossref] [PubMed]
- Topilsky Y, Maltais S, Medina Inojosa J, et al. Burden of Tricuspid Regurgitation in Patients Diagnosed in the Community Setting. JACC Cardiovasc Imaging 2019;12:433-42. [Crossref] [PubMed]
- Fender EA, Zack CJ, Nishimura RA. Isolated tricuspid regurgitation: outcomes and therapeutic interventions. Heart 2018;104:798-806. [Crossref] [PubMed]
- Taramasso M, Gavazzoni M, Pozzoli A, et al. Tricuspid Regurgitation: Predicting the Need for Intervention, Procedural Success, and Recurrence of Disease. JACC Cardiovasc Imaging 2019;12:605-21. [Crossref] [PubMed]
- Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol 2004;43:405-9. [Crossref] [PubMed]
- Chorin E, Rozenbaum Z, Topilsky Y, et al. Tricuspid regurgitation and long-term clinical outcomes. Eur Heart J Cardiovasc Imaging 2020;21:157-65. [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:e521-643. [PubMed]
- Kilic A, Saha-Chaudhuri P, Rankin JS, et al. Trends and outcomes of tricuspid valve surgery in North America: an analysis of more than 50,000 patients from the Society of Thoracic Surgeons database. Ann Thorac Surg 2013;96:1546-52; discussion 52. [Crossref] [PubMed]
- Lama P, Tamang BK, Kulkarni J. Morphometry and aberrant morphology of the adult human tricuspid valve leaflets. Anat Sci Int 2016;91:143-50. [Crossref] [PubMed]
- Fukuda S, Saracino G, Matsumura Y, et al. Three-dimensional geometry of the tricuspid annulus in healthy subjects and in patients with functional tricuspid regurgitation: a real-time, 3-dimensional echocardiographic study. Circulation 2006;114:I492-8. [Crossref] [PubMed]
- Ton-Nu TT, Levine RA, Handschumacher MD, et al. Geometric determinants of functional tricuspid regurgitation: insights from 3-dimensional echocardiography. Circulation 2006;114:143-9. [Crossref] [PubMed]
- Hahn RT. State-of-the-Art Review of Echocardiographic Imaging in the Evaluation and Treatment of Functional Tricuspid Regurgitation. Circ Cardiovasc Imaging 2016.9. [PubMed]
- Pluchinotta SPSaFR. Tricuspid Valve: Embryology and Anatomy. In: Allsessandro Giamberti MC, editor. The Tricuspid Valve in Congenital Heart Disease. New York: Springer, 2014:1-13.
- Mahmood F, Kim H, Chaudary B, et al. Tricuspid annular geometry: a three-dimensional transesophageal echocardiographic study. J Cardiothorac Vasc Anesth 2013;27:639-46. [Crossref] [PubMed]
- Spinner EM, Shannon P, Buice D, et al. In vitro characterization of the mechanisms responsible for functional tricuspid regurgitation. Circulation 2011;124:920-9. [Crossref] [PubMed]
- Topilsky Y, Khanna A, Le Tourneau T, et al. Clinical context and mechanism of functional tricuspid regurgitation in patients with and without pulmonary hypertension. Circ Cardiovasc Imaging 2012;5:314-23. [Crossref] [PubMed]
- Muraru D, Hahn RT, Soliman OI, et al. 3-Dimensional Echocardiography in Imaging the Tricuspid Valve. JACC Cardiovasc Imaging 2019;12:500-15. [Crossref] [PubMed]
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685-713. [Crossref] [PubMed]
- Addetia K, Yamat M, Mediratta A, et al. Comprehensive Two-Dimensional Interrogation of the Tricuspid Valve Using Knowledge Derived from Three-Dimensional Echocardiography. J Am Soc Echocardiogr 2016;29:74-82. [Crossref] [PubMed]
- Hahn RT, Abraham T, Adams MS, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2013;26:921-64. [Crossref] [PubMed]
- Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017;30:303-71. [Crossref] [PubMed]
- Lancellotti P, Moura L, Pierard LA, et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr 2010;11:307-32. [Crossref] [PubMed]
- Rivera JM, Mele D, Vandervoort PM, et al. Effective regurgitant orifice area in tricuspid regurgitation: clinical implementation and follow-up study. Am Heart J 1994;128:927-33. [Crossref] [PubMed]
- Topilsky Y, Nkomo VT, Vatury O, et al. Clinical outcome of isolated tricuspid regurgitation. JACC Cardiovasc Imaging 2014;7:1185-94. [Crossref] [PubMed]
- de Agustin JA, Viliani D, Vieira C, et al. Proximal isovelocity surface area by single-beat three-dimensional color Doppler echocardiography applied for tricuspid regurgitation quantification. J Am Soc Echocardiogr 2013;26:1063-72. [Crossref] [PubMed]
- Utsunomiya H, Harada Y, Susawa H, et al. Comprehensive Evaluation of Tricuspid Regurgitation Location and Severity Using Vena Contracta Analysis: A Color Doppler Three-Dimensional Transesophageal Echocardiographic Study. J Am Soc Echocardiogr 2019;32:1526-37.e2. [Crossref] [PubMed]
- Velayudhan DE, Brown TM, Nanda NC, et al. Quantification of tricuspid regurgitation by live three-dimensional transthoracic echocardiographic measurements of vena contracta area. Echocardiography 2006;23:793-800. [Crossref] [PubMed]
- Chen TE, Kwon SH, Enriquez-Sarano M, et al. Three-dimensional color Doppler echocardiographic quantification of tricuspid regurgitation orifice area: comparison with conventional two-dimensional measures. J Am Soc Echocardiogr 2013;26:1143-52. [Crossref] [PubMed]
- Song JM, Jang MK, Choi YS, et al. The vena contracta in functional tricuspid regurgitation: a real-time three-dimensional color Doppler echocardiography study. J Am Soc Echocardiogr 2011;24:663-70. [Crossref] [PubMed]
- Hahn RT, Zamorano JL. The need for a new tricuspid regurgitation grading scheme. Eur Heart J Cardiovasc Imaging 2017;18:1342-3. [Crossref] [PubMed]


