
Stereotactic ablative radiotherapy for malignant mediastinal and hilar lymphadenopathy: a systematic review
Introduction
Stereotactic ablative body radiotherapy (SABR), also known as stereotactic body radiotherapy (SBRT) is a form of hypofractionated radiotherapy (RT), typically delivered in ≤5 fractions (1,2). Since its adoption into clinical practice, SABR has evolved to have a wide range of clinical applications, from curative-intent treatment for primary lesions such as in early stage non-small cell lung cancer (NSCLC) to controlling oligometastatic lesions in various organ targets such as the liver, adrenal and spine (3,4).
Mediastinal and hilar lymphadenopathy (MHL) is a frequent pattern of cancer spread, especially in, but not limited to, primary lung malignancies (5,6). Local treatments have traditionally included surgical resection or conventional RT, with or without systemic treatments (7). Recently, there has been interest in the application of SABR for MHL, especially in the oligometastatic setting, to improve local control (LC) and achieve shorter treatment durations to minimize systemic treatment breaks (4,8,9). However, the proximity of MHL to critical structures such as the esophagus, trachea, proximal bronchial tree (PBT), great vessels and heart may compromise the optimal delivery of SABR due to organ dose constraints (9,10). This is analogous to “ultracentral” lung tumors, commonly defined as lesions with gross tumor volumes (GTV) in contact with PBT and/or other mediastinal structures such as great vessels and esophagus, and are the subject of ongoing investigations (11,12).
To our knowledge, there is no published prospective randomized data on SABR for MHL. Given the lack of level 1 evidence, the purpose of this manuscript is to systematically review the published experience of SABR for MHL to date. Specifically, this study will summarize outcomes of MHL SABR such as LC, progression free survival (PFS) and overall survival (OS), as well as toxicity, with a focus on MHL SABR-related fatalities (grade 5 toxicity).
Methods
A systematic review based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines was performed using MEDLINE® (PubMed®), EMBASE and Cochrane Library databases from inception until December 2018 (13). Our search strategy aimed to include studies containing patients with mediastinal or intrathoracic lymphadenopathies from all primary malignancies and investigated SABR for cancer therapies. For this study, SABR was defined as hypofractionated RT (≥5 Gy/fraction) delivered using stereotactic techniques (e.g., high dose heterogeneity with patient immobilization). The exact search terms can be found in the Supplemental materials.
The following criteria were used for inclusion in the systematic review:
- Population: cancer patients with mediastinal and/or hilar metastatic lymphadenopathies.
- Intervention: SABR to the MHL.
- Comparison: none.
- Outcomes: MHL SABR-related toxicities and mortalities. LC, OS, and PFS if available.
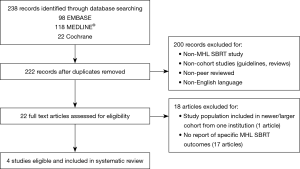
Two investigators independently screened the titles and abstracts of all retrieved records, with discrepancies settled by a third investigator. Non-English studies, guidelines, reviews, non-peer reviewed correspondences were excluded. Only studies reporting on SABR specifically for MHL were included. If multiple publications were found from the same institution, only the most recent publication and/or largest cohort were included for data abstraction. The screening process is illustrated in Figure 1. Journal articles that met all criteria on abstract and full text review underwent data extraction. Extracted data were confirmed independently by two investigators. Data extracted from studies included (if available): study factors (design, sample size); patient demographics (age, sex); cancer details (primary tumor, number and location of lymph nodes); treatment details (prior overlapping radiation, prior systemic therapy, RT dose fractionation, technical details, target volumes); outcome data (follow-up duration, LC, OS, PFS); and finally toxicity.
As a variety of dose fractionation schedules were employed, we converted these to a standardized Biologically effective dose (BEDα/β) was calculated using the following formula:
Results
Study and patient characteristics
Two hundred and twenty-two abstracts were identified by the literature search and 22 studies underwent full-text assessment after preliminary screening. Four studies with a total of 196 patients were included in our final analysis. Three studies (study 2–4) published between 2016 and 2018 met all inclusion criteria, and a fourth study (study 1) with a small subset of patients receiving non-ablative hypofractionated RT doses (<5 Gy per fraction) delivered using stereotactic technique. We included study 1 despite inclusion of patients receiving stereotactic hypofractionated RT as out of 98 MHL treated, only a minority of cases in stations 5 (n=17) and 7 (n=9) received such regimens. Median dose per fraction to station 5 was 8.4 [4.5–10] Gy, and station 7 was 6 [3–9] Gy. Given the majority patients in study 1 received SABR doses, important toxicity findings in the study, and due to the small number of studies identified reporting SABR for MHL, we decided to include study 1 in our analysis.
Study selection process was illustrated in Figure 1 (5,9,14,15). All were retrospective cohort studies. Median patient age ranged from 59 to 70 years. The majority (65%) of patients (n=127) had a diagnosis of NSCLC; and breast was the second most common primary tumor with 16 patients (8%). Sixty-three (32%) patients had prior overlapping thoracic RT and 92 (47%) patients had prior systemic therapies, including chemotherapies, targeted therapies, and hormonal therapies. Notably, there were no reports of anti-vascular endothelial growth factor (VEGF) agents, nor was there information on the use of anticoagulation therapies.
All patients had Eastern Cooperative Oncology Group (ECOG) performance scores between 0 and 2. Post-treatment surveillance was performed using CT imaging in all studies, with 18-FDG-PET scan to confirm recurrences in study 1–3. Median follow-up period ranged from 12 to 32 months. Full study details are summarized in Table 1.

Full table
SABR technical details
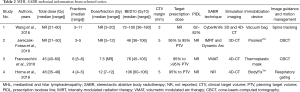
Across 4 studies, the total SABR and stereotactic hypofractionated RT doses and fractions ranged from 21 to 60 Gy in 3 to 11 fractions (dose per fraction between 3–12 Gy); the BED10 ranged from 36 to 180 Gy10. Study 1 reported BED10 median for each MHL nodal station, median ranges were: 72–86 Gy10 for station 1–4, 77–82 Gy10 for station 5–6, 77–94 Gy10 for station 8–9, and 83–100 Gy10 for station 10. Overall, BED10 medians of studies 1–4 ranged between 46 and 106 Gy10. Studies 1, 3, and 4 incorporated 4-dimensional (4D)-CT during simulation, with study 2 using only free-breathing 3D-CT-simulation technique; all used immobilization devices. Study 3 also incorporated PET for target delineation and planning for all cases, while study 1 only for some cases. Cone beam-CT (CBCT) was used for image guidance during treatment in study 2 and 3, while spine tracking-system was used in study 1. Study 4 used respiratory gating for patients with tumor motion >0.5 cm.
A margin of 5 mm was applied to nodal CTV in studies 2–4, while study 1 applied a 3 mm margin. Study 1 reported a prescription to the 62% to 82% isodose line and studies 2–4 aimed for at least 95% of the planning target volume (PTV) to receive 95% of the prescribed dose. RT technical details are summarized in Table 2.
Full table
LC
Three studies reported LC rates: study 1 reported 97% and 77% at 1 and 5-years, respectively, study 2 reported 69% and 66% at 6 and 16-months, respectively, and study 4 reported 88% at 2-year (Table 3).

Full table
Toxicities
All studies reported grade 3–5 toxicities using the Common Terminology Criteria for Adverse Events (CTCAE, v4.0). A total of 11 (6%) patients experienced grade ≥3 toxicities. There were 4 (2%) treatment-related, grade 5, toxicities reported. Study 1 reported 3 fatalities: tracheo-esophageal fistulae [2] and esophageal-mediastinal fistula [1] (Table 3). These patients all had prior RT to station 7 lymph nodes. Study 1 provided details of one grade 5 tracheo-esophageal fistula case in a patient who had definitive RT (dose unknown) covering station 7 node 6.8 months prior presenting with station 7 node recurrence. Prescription dose was 48 Gy in 8 fractions to 75% isodose line covering 95% PTV. Tracheal and esophageal Dmax were 61.8 and 52.9 Gy. No SABR details were provided in other 2 grade 5 cases. Study 4 reported 1 fatality due to heart failure after MHL SABR in a patient with a significant coronary disease (previous quadruple bypass surgery) and congestive heart failure. There were 3 grade 4 toxicities: hemoptysis [1], pericardial and pleural effusion [1], and myocardial infarction [1]. There were 4 grade 3 toxicities: tracheitis [2], pneumonitis [1], and esophagitis [1].
Other outcomes
Reported OS values from study 1, 3, and 4 ranged from 18–27 months. One-year OS rates ranged from 69–76%. In studies 3 and 4, median PFS were 13 and 9 months, respectively and 1-year PFS were 54% and 28%, respectively.
Discussion
In this systematic review of MHL SABR a limited number of retrospective single-institutional cohort studies were identified and were heterogeneous both in the RT doses employed and endpoints reported. The available data herein suggests that MHL SABR is feasible, and while generally well tolerated, is uncommonly associated with serious and potentially treatment-related grade 5 toxicity. Specific caution is warranted in the re-irradiation setting. As MHL SABR is increasingly being considered in broader radiation oncology practice, prospective studies that allow for its utilization (i.e., protocols of SABR in oligometastatic treatment) will help clarify its efficacy and safety profiles. Questions that warrant further data include the most appropriate dose fractionation schedules, integration within developments of systemic therapy (including targeted therapies and immunotherapies), and clarification of dose volume constraints risk profiles for organs at risk.
With a pooled grade ≥3 toxicity rate of 6%, MHL SABR seemed to be relatively safe, though the 2% rate of SABR-related fatalities highlights the need to exercise caution. Three of these toxicities were tracheo-esophageal or esophageal-mediastinal fistulae which occurred in patients with previous RT for station 7 (subcarinal) lymphadenopathies, an anatomic location adjacent to esophagus and trachea (9). Anatomically, the scenario of MHL SABR is similar to the use of SABR for ultra-central lung tumors, which is defined as tumor abutting PBT and/or other mediastinal structures (e.g., great vessels and esophagus) (12). A recently published systematic review containing 10 retrospective studies examined the safety and efficacy of SABR for ultracentral lung lesions, although MHL SABR was not included in 9 of the studies (12). From this review, the reported median grade ≥3 toxicity rate from studies was 10% and the median treatment-related fatality rate of 5% (12). Ultimately, 4 high-risk scenarios of ultra-central lung SABR were described as follows: (I) elevated maximum dose to the PBT, (II) endobronchial disease, (III) use of anti-VEGF agents such as bevacizumab, and (IV) use of anticoagulants. We would contend that these in addition to the consideration of prior RT, as identified in the present study, would serve indicators of higher-risk scenarios for MHL SABR. There was no reported use of anti-VEGF agents in studies included for our analysis; given the available knowledge, we would suggest that caution is warranted when considering MHL SABR, where there is recent or planned anti-VEGF use.
To guide the RT planning objectives of MHL SABR, various protocols and guidelines can be considered. The UK SABR Consortium has proposed mandatory Dmax (0.5 cc) constraints for 3, 5 and 8 fraction SABR for thoracic OARs, and suggested 32, 35, and 44 Gy respectively for the PBT, 25.2, 34, and 40 Gy respectively for the esophagus, and 45 and 53 Gy in 3 and 5 fractions respectively for the great vessels (10,16). The case of a fatal tracheo-esophageal fistula in a patient with prior station 7 node RT in study 1 showed tracheal and esophageal Dmax of 61.8 and 52.9 Gy in 8 fractions, respectively. This highlights the importance of awareness of OAR constraints, especially in re-irradiation settings. The recently reported RTOG 0813 phase I/II trial, which investigated safety and efficacy SABR in stage I NSCLC with central tumors, concluded that 60 Gy in 5 fractions was achievable, with an acceptable dose limiting toxicity of 7.2% (17). In this prospective study, central was defined as tumors within 2 cm of the PBT, as well as those adjacent to the mediastinum and pericardium. We would indicate that MHL SABR would incur a higher risk scenario, owing to multi-target potential overlap with central structures within the mediastinum, and would therefore caution against a dose fractionation as high as 60 Gy in 5 fractions in this setting.
In terms of actively accruing prospective studies, NRG LU002 (ClinicalTrials.gov ID: NCT03137771) is a randomized controlled trial of synchronous oligometastatic NSCLC patients, comparing standard of care systemic therapy with or without local consolidative treatment, and allows for MHL SABR. The protocol from this cooperative group trial recommends a D0.03 cc and D5 cc of 40 and 32 Gy in 5 fractions, respectively, for the PBT and values of 35 and 19.5 Gy in 5 fractions, respectively, for the esophagus. While toxicity with MHL SABR appears to be uncommon, our current review suggests that MHL-SABR toxicity may be comparable to that of cEBRT. Recognizing the caveats of comparing different retrospective studies, reported MHL-cEBRT grade ≥3 toxicities range from 0 to 8%, and treatment related fatalities are in the range of 0 to 4% (18-20). Ultimately, the risk of toxicity may relate to the volume overlap and/or proximity of PTV and a relevant OAR. As a result, there are competing risks between optimizing LC and minimizing toxicity, which raises the question of the relative therapeutic ratios of SABR versus hypofractionation, as demonstrated in a planning study by Murrell et al. (21).
There is lack of consistency in the reporting of LC post-MHL SABR hindering proper pooling of the outcome, with study 1 reported LC at 1 and 5 years (97% and 77% respectively), study 4 reported LC at 2 years (88%), while study 2 reported LC at 6 and 16 months (69% and 66% respectively). The poor LC reported by study 2 might be due to a lower median BED10 (46 Gy10) used in MHL SABR compared to the other 3 studies (median BED10 >70 Gy10). Given the dose-response relationship between BED10 and clinical outcomes in primary lung cancer, perhaps a similar relationship exists for MHL (22,23). Other factors such as the lymph node stations targeted or previous RT could also have impacted LC, though there is insufficient data in the included studies to draw meaningful inferences. Comparatively, reported historical LC rates of MHL treated with conventional external beam radiotherapy (cEBRT) (50–84 Gy total, 2–3 Gy per fraction, delivered without high intra-target dose heterogeneity) have been reported at 76–88% at 1 year, 76% at 2 years, and 61% at 5 years post-treatment (18-20). Based on data included in the current systematic review, MHL SABR seemed to yield comparable LC to cEBRT with shorter treatment period [3–11 daily fractions with SABR, compared to typically ≥30 fractions with cEBRT (18,20)].
This systematic review should be considered in the context of both its strengths and limitations. As this is a relatively newer application of SABR, all 4 studies were relatively small (largest: 85 patients) and retrospective in nature, with inherent biases of both treatment indication as well as classification of non-objective outcomes such as toxicity. There were a variety of dose fractionation schemes employed, most with a calculated BED10 significantly lower than 100 Gy10 likely due to concerns related toxicity. Furthermore, 1 study included both SABR and less hypofractionated schemes, which could result in an underestimation of LC and adverse events described. The majority of patients had a primary diagnosis of NSCLC and, therefore the generalizability of MHL within other primary histologies is unclear, especially as types of systemic therapy used in other metastatic diseases may differ (4,8). Given the uncertainties associated with MHL SABR described, its relative merits of convenience need to be considered against its potential risks, as further larger studies, both prospective and retrospective in nature are awaited.
Supplementary
Systematic review search strategies
MEDLINE® (PubMed®)
(sabr[tw] OR sbrt[tw] OR srt[tw] OR stereotactic[tw] OR radiosurgery[mh])
AND
(mediastinum[tw] OR mediastinum[mh] OR mediastinal[tw] OR thoracic[tw] OR thorax[mh] OR mediastinal neoplasms[mh])
AND
(node[tw] or nodal[tw] OR nodes[tw] OR lymph nodes[mh])
Results: 118
EMBASE
(sabr.mp. or sbrt.mp. or exp stereotactic body radiation therapy/ or exp stereotactic radiosurgery/ or srt.mp. or stereotactic.mp. or radiosurgery.mp. or exp radiosurgery/ or exp gamma knife radiosurgery/)
and
(exp mediastinum/ or mediastinum.mp. or mediastinal.mp. or thoracic.mp. or exp thorax/ or thorax.mp. or exp mediastinum tumor/)
and
(node.mp. or exp lymph node/ or nodes.mp. or nodal.mp.)
Limit: exclude medline journals
Results: 98
Cochrane Library
(sabr or sbrt or srt or stereotactic or radiosurgery)
and
(mediastinum or mediastinal or thoracic or thorax)
and
(node or nodal or nodes or lymph node)
Results 22
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.112). AVL reports personal fees from AstraZeneca, personal fees from Varian Medical Systems Inc., outside the submitted work; serves as an unpaid editorial board member of Journal of Thoracic Disease from Nov 2018 to Nov 2020. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Goldsmith C, Gaya A. Stereotactic ablative body radiotherapy (SABR) for primary and secondary lung tumours. Cancer Imaging 2012;12:351-60. [Crossref] [PubMed]
- Senthi S, Haasbeek CJA, Slotman BJ, et al. Outcomes of stereotactic ablative radiotherapy for central lung tumours: A systematic review. Radiother Oncol 2013;106:276-82. [Crossref] [PubMed]
- Palma DA, Louie A V, Rodrigues GB. New strategies in stereotactic radiotherapy for oligometastases. Clin. Cancer Res 2015;21:5198-204. [Crossref] [PubMed]
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet 2019;393:2051-8. [Crossref] [PubMed]
- Franceschini D, De Rose F, Fogliata A, et al. Volumetric modulated arc therapy for thoracic node metastases: a safe and effective treatment for a neglected disease. Oncotarget 2016;7:53321-9. [Crossref] [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the TNM Stage Groupings in the Forthcoming (Seventh) Edition of the TNM Classification of Malignant Tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Dickhoff C, Dahele M, Smit EF, et al. Patterns of care and outcomes for stage IIIB non-small cell lung cancer in the TNM-7 era: Results from the Netherlands Cancer Registry. Lung Cancer 2017;110:14-8. [Crossref] [PubMed]
- Gomez DR, Tang C, Zhang J, et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J Clin Oncol 2019;37:1558-65. [Crossref] [PubMed]
- Wang HH, Zaorsky NG, Meng MB, et al. Stereotactic radiation therapy for oligometastases or oligorecurrence within mediastinal lymph nodes. Oncotarget 2016;7:18135-45. [Crossref] [PubMed]
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive Toxicity When Treating Central Tumors in a Phase II Study of Stereotactic Body Radiation Therapy for Medically Inoperable Early-Stage Lung Cancer. J Clin Oncol 2006;24:4833-9. [Crossref] [PubMed]
- Palma D, Daly M, Urbanic J, et al. Stereotactic Radiation for Ultra-Central Lung Tumors: Good Idea, or Ultra-Risky? Int J Radiat Oncol 2019;103:788-91. [Crossref] [PubMed]
- Chen H, Laba JM, Zayed S, et al. Safety and Effectiveness of Stereotactic Ablative Radiotherapy for Ultra-Central Lung Lesions: A Systematic Review. J Thorac Oncol 2019;14:1332-42. [Crossref] [PubMed]
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1-34. [Crossref] [PubMed]
- Horne ZD, Richman AH, Dohopolski MJ, et al. Stereotactic body radiation therapy for isolated hilar and mediastinal non-small cell lung cancers. Lung Cancer 2018;115:1-4. [Crossref] [PubMed]
- Jereczek-Fossa BA, Muto M, Durante S, et al. Stereotactic body radiation therapy for mediastinal lymph node metastases: how do we fly in a ‘no-fly zone’? Acta Oncol 2018;57:1532-9. [Crossref] [PubMed]
- Hanna GG, Murray L, Patel R, et al. UK Consensus on Normal Tissue Dose Constraints for Stereotactic Radiotherapy. Clin Oncol (R Coll Radiol) 2018;30:5-14. [Crossref] [PubMed]
- Bezjak A, Paulus R, Gaspar LE, et al. Safety and efficacy of a five-fraction stereotactic body radiotherapy schedule for centrally located non-small-cell lung cancer: NRG Oncology/RTOG 0813 trial. J Clin Oncol 2019;37:1316-25. [Crossref] [PubMed]
- Manabe Y, Shibamoto Y, Baba F, et al. Radiotherapy for hilar or mediastinal lymph node metastases after definitive treatment with stereotactic body radiotherapy or surgery for stage I non-small cell lung cancer. Pract Radiat Oncol 2012;2:e137-43. [Crossref] [PubMed]
- Okami J, Nishiyama K, Fujiwara A, et al. Radiotherapy for Postoperative Thoracic Lymph Node Recurrence of Non-Small-Cell Lung Cancer Provides Better Outcomes If the Disease Is Asymptomatic and a Single-Station Involvement. J Thorac Oncol 2013;8:1417-24. [Crossref] [PubMed]
- Seol KH, Lee JE, Cho JY, et al. Salvage radiotherapy for regional lymph node oligo-recurrence after radical surgery of non-small cell lung cancer. Thorac Cancer 2017;8:620-9. [Crossref] [PubMed]
- Murrell DH, Laba JM, Erickson A, et al. Stereotactic ablative radiotherapy for ultra-central lung tumors: Prioritize target coverage or organs at risk? Radiat Oncol 2018;13:57. [Crossref] [PubMed]
- Kestin L, Grills I, Guckenberger M, et al. Dose-response relationship with clinical outcome for lung stereotactic body radiotherapy (SBRT) delivered via online image guidance. Radiother Oncol 2014;110:499-504. [Crossref] [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Hypofractionated Stereotactic Radiotherapy (HypoFXSRT) for Stage I Non-small Cell Lung Cancer: Updated Results of 257 Patients in a Japanese Multi-institutional Study. J Thorac Oncol 2007;2:S94-100. [Crossref] [PubMed]


