Is pulmonary vein isolation still the cornerstone in atrial fibrillation ablation?
Atrial fibrillation (AF) is the most common cardiac arrhythmia encountered in clinical practice with significant morbidity and mortality. Radiofrequency catheter ablation for AF has become a common used second-line therapy after failure of at least one antiarrhythmic drug. Yet, given the outcomes with catheter ablation there is increasing interest in its use as first-line therapy particularly in certain subgroups of patients. The basis for catheter ablation as a first-line therapy in AF is derived from an increasing body of research demonstrating superiority of catheter ablation over antiarrhythmic drug therapy in maintaining sinus rhythm. For example, multiple clinical trials report arrhythmia free survival of 50-75% at 1-year post ablation in contrast to only 10-30% with antiarrhythmic drugs (1-6). Recent studies have shed new light on both clinical and subclinical outcomes through expanded monitoring and increased length of follow-up. Unfortunately, the differences in outcomes between catheter ablation and medications that prompted tremendous initial enthusiasm appear to be worse than previously reported (7,8). Many of these latter studies examined catheter ablation in patients naive to an antiarrhythmic drug therapy and as such studied therapies earlier in the disease process. Recently, we demonstrated that arrhythmia-free survival rates with catheter ablation are critically dependent on the delays from the initial arrhythmia diagnosis to the procedure (9). However, medications used early in the disease process are also typically much more effective. As such, the difference between outcomes becomes less clear, particularly during a relatively short follow-up of 1-2 years. Understanding these success rates and their temporal evolution with aging requires a review and assessment of the fundamental cornerstone of catheter ablation for AF, durable pulmonary vein isolation.
Pulmonary vein triggers
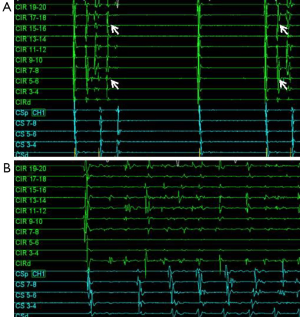
In 1998, Haïssaguerre et al. (10) showed that pulmonary vein ectopy can trigger AF and electrical isolation of these pulmonary vein triggers can suppress AF. They found that in 29 patients there was a single point of origin for the AF, whereas two points were identified in nine patients, and 3-4 points in another seven patients. Of these ectopic sites, 94% originated in the pulmonary veins. Figure 1 shows an example of a repetitive single pulmonary vein trigger resulting in AF. The study by Haïssaguerre and colleagues heralded the current era of non-pharmacologic treatment approaches to AF management, which are founded on pulmonary vein isolation.
Initial isolation approaches focused on identifying specific pulmonary vein triggers utilizing induction strategies. These pulmonary vein triggers were then targeted with segmental isolation (10,11). Unfortunately an induction strategy as a stepwise approach to pulmonary vein isolation was challenging due to difficulty in initiating the arrhythmogenic foci during the electrophysiology study. In addition, during subsequent studies in patients with AF recurrence, different ectopic foci from the same or different pulmonary veins were often found to be active contributors to the arrhythmia. As such, empiric isolation of all pulmonary veins became the preferred strategy (12,13). However, it is important to recognize that a close examination of long-term outcomes does not demonstrate that empiric isolation of all pulmonary veins is superior to treating only those veins that are arrhythmogenic (14).
Based upon the initial work by Haïssaguerre et al. (10) long-term success rates with pulmonary vein isolation alone should be much higher. For example, in the initial studies not all identified triggers were from the pulmonary veins, but over 90% were. However, success rates after catheter ablation were significantly less than 90% at 1 year. Furthermore, in studies with follow-up to 3-5 years success rates are even lower at 30-50% (15,16). In non-paroxysmal AF, recurrence rates are also very high with recurrence rates of 70% at 3 years despite aggressive ablation approaches (17). These long-term success rates should prompt a careful inquiry into why catheter ablation for AF is fraught with such high recurrence rates. Failure may stem from inadequate initial therapy or it may reflect an inadequate understanding of AF and how to utilize nonpharmaceutic therapy.
Achieving durable pulmonary vein isolation
One basic assumption made when raising the question if pulmonary vein isolation is enough for a “cure” or durable therapy for AF is that the index ablation procedure resulted in permanent isolation of the pulmonary veins. In a long-term follow-up study that investigated the rate of pulmonary vein reconnection after initial isolation, 53% of 161 patients were free of AF. In 66 patients, a repeat ablation was performed for repeat arrhythmia. The rate of pulmonary vein reconnection was strikingly high at 94% (62 of 66 patients) (15). The importance of pulmonary vein reconnection has been confirmed in other studies and has led to the postulate that electrical reconnection of the veins is an important mechanism of AF recurrence following catheter ablation (16,18,19).
This raises the question of why durable pulmonary vein isolation with catheter ablation is so difficult. There are several potential mechanisms that may underlie pulmonary vein reconnection. First, it is possible that the initial procedure failed to achieve complete electrical isolation of the pulmonary vein. Incomplete isolation is felt to result from residual gap(s) within the encircling lesion set or lack of transmural lesions (20,21). As such, it is could be postulated that early recurrence of AF post ablation may be an early marker of incomplete procedural pulmonary vein isolation. This hypothesis is supported by an interesting study of 12 patients that underwent a maze procedure after a failed radiofrequency ablation. Importantly, myocardial biopsies showed anatomic gaps and/or nontransmural lesions in pulmonary veins that had reconnected (22) Enhanced post-procedural imaging has also added further supported to this hypothesis. In a canine study in which endocardial conduction block was demonstrated, post procedural MRI identified gaps within the line of ablation. Finally, long-term follow up data has demonstrated that those pulmonary veins with MRI identified gaps were more likely to become electrically reconnected with symptomatic recurrences (21).
Another potential mechanism for pulmonary vein reconnection involves the early post ablation management of the patient and its potential impact on atrial remodeling. For example, therapies such as an early strategy of cardioversion and colchicine have been shown to significantly improve long-term arrhythmia-free survival (23-25). Inflammation is an important mechanism for post-op AF and may be an important mechanism for early recurrence of AF post ablation. For example, it is likely that inflammation post ablation adversely effects atrial remodeling through complex mechanisms. Thus, it could be postulated that therapies that target inflammatory pathways such as colchicine may favorably impact post ablation atrial remodeling. However, it should be noted that during inflammation fibroblasts are recruited to an injury site and stimulate the scar formation that is essential for durable lesion formation. As such, it is possible that approaches to minimize post-procedure inflammation could be deleterious. There are data to suggest that cardiac specific stem cells are also recruited to the regions of injury. These cells can differentiate into myofibroblasts or myocytes capable of electrical conduction. Though this mechanism has not been demonstrated with pulmonary vein isolation, it has been shown in donor-mismatch patients with local inflammation and injury stemming from coronary artery disease (26). As such, therapies that reduce inflammation to minimize the recruitment of additional drivers, but do not adversely effect durable scar formation may enhance the durability of pulmonary vein isolation procedures. In this manner cardioversion to sinus rhythm to reduce rate-dependent atrial myopathy may provide benefit.
It is also possible that pulmonary vein isolation at the antrum is insufficient to completely isolate the pulmonary vein triggers as they can find other routes of atrial activation from adjacent venous structures such as the superior vena cava, coronary sinus, or ligament of Marshall/Marshall vein (27,28). We suspect that this mechanism is likely less of a contributor to recurrences since most of the repeat ablation procedures show recurrence of pulmonary vein conduction with overt loss of the previously achieved entrance/exit block. However, these other avenues of conduction or triggered activity may still be important as additional ablation of the ligament of Marshall, coronary sinus, and superior vena cava has been shown to improve ablation success. Unfortunately, it is not known fully if this is because these veins are independent triggers of arrhythmia, conduits of arrhythmia triggers from other sources, or a combination of both.
Examining outcomes with suboptimal pulmonary vein isolation
Although pulmonary vein reconnection occurs in the majority of patients with AF recurrence post ablation, recent data suggests that pulmonary vein reconnection is also very common among patient that remain arrhythmia-free. This was highlighted in an interesting study of 32 patients without clinical recurrence of AF in which a repeat electrophysiological study was performed. In this study recovery of pulmonary vein conduction was observed in 90.6% of patients. In fact, 31.2% of these AF patients had reconnection of all four veins and another 21.9% had reconnection in 3 of 4 veins (29) These data certainly highlight the need for further investigation to clarify the mechanistic role of electrical reconnection of pulmonary veins on recurrence of AF post ablation.
In further support of the lack of pulmonary vein isolation impact on those patients without symptomatic recurrences a recent study compared contrast MRI scar imaging with scar that was predicted by the 3D voltage maps during the ablation procedure (30). In this study, the mean percentage of scar quantified by electroanatomic mapping was 30.5%±7.5% compared to only 13.9%±5.9% quantified by MRI. There are several mechanisms that may be behind these discrepant findings. First, electroanatomic maps are dependent on ideal catheter-tip and atrial wall contact. In the absence of adequate contact, local electrical signal strength can be lost or diminished and appear erroneously as scar. Second, electroanatomic maps are dependent on sampling. Point-by-point area samples are then integrated to project a 3D image with algorithmic delineation of tissue characteristics such as scar. If one or more of these points is misclassified, then the region is also prone to misclassification error. Errors are typically a product of points sampled, since intra-point automated interpolation is smaller. Thereby, dense maps containing multiple sampling sites with good catheter tip to tissue contact will be more accurate than less dense maps. Third, delivery of ablative energy does not correlate with a transmural lesion and definitive scar formation. In this case the electroanatomic map may be correct, but the durable injury per lesion marker is less than anticipated. Means to improve transmural lesion formation will be discussed subsequently the topic how to improve durable pulmonary vein isolation is developed. In regards to the trial that found significant discord between electroanatomic map based scar quantification and that observe by cardiac MRI, the authors did not report their long-term arrhythmia-free success rates. However, a contemporary article from the same institution of patients that underwent an AF ablation and received a cardiac MRI reported a 1-year survival free of atrial arrhythmia rate post ablation of 69.1% (31). Summarizing these two trials it is reasonable to assume patients with very little scar post ablation around the pulmonary veins had no symptomatic recurrence. These studies raise some very important questions about our assumptions of pulmonary vein antral isolation with catheter ablation. First, is the amount of myocardial tissue injury necessary to produce acute pulmonary vein isolation adequate to produce durable scar. If not, what markers other than acute pulmonary vein isolation can be used to suggest that adequate ablation has been performed to produce durable scar around the antra of the pulmonary veins. Based on the findings of these studies it seems that the amount of durable scar produced during the majority of pulmonary vein antral isolation is much less than predicted. Also, continuous transmural lesions around the pulmonary veins seem to be rare. As such, pulmonary vein isolation confirmed by exit and entrance block is likely an insufficient guide. Finally, the lack of MRI identified scar and lack of durable pulmonary vein isolation did not seem to affect the outcomes. Therefore is pulmonary vein isolation the cornerstone of ablation procedures or it some other mechanism targeted during the ablation the driver of success?
These studies do highlight an important concept, without durable pulmonary vein isolation, it is difficult to draw strong conclusions regarding its’ significant in the long-term arrhythmia outcomes after AF ablation. This is particularly important in that we have mechanistic evidence of pulmonary vein triggers in patients with paroxysmal AF (Figure 1).
Improving durable pulmonary vein isolation
Currently, there are no proven ways to improve long-term durable pulmonary vein isolation, largely because we are just beginning to realize the frequency of reconnection in patients without recurrence of arrhythmia. In the few studies available that have investigated the presence or absence pulmonary vein isolation in patients without recurrent AF during subsequent electrophysiology study, the reconnection rates range from 0-90% (29,32,33). Thereby our understanding of reconnection rates and their significance has been primarily derived from patients with a failed prior ablation that present for a subsequent electrophysiology study.
There are several technologies and approaches that have been advocated to improve the likelihood of transmural lesion formation and durable pulmonary vein isolation. The efficacy of these technologies is largely based upon periprocedural data with the previously noted limitations, but hopefully acute or periprocedural results will translate to enhanced long-term outcomes. Multi-pore irrigated tip catheter technologies that enhance energy delivery efficiency result in lower periprocedural reconnection rates compared to standard irrigated tip catheters (34). Contact force sensing catheters can provide continuous feedback regarding catheter contact and stability. Maintaining a contact force of >10 grams is associated with a lower likelihood of pulmonary vein reconnection during adenosine provocation after initial pulmonary vein isolation (35). In a study that examined pulmonary vein reconnection rates between standard radiofrequency approaches vs. cryoballoon therapy there was no significant differences in reconnection rates found in those patients that presented for repeat ablation despite lower procedure times and small troponin elevations in the cryothermal treated patients (36).
Regarding procedural approaches, one of the most efficient ways to look for pulmonary vein reconnection is to increase the peri-procedural observation. In a study of 181 patients, waiting 35 minutes after acute isolation to examine for reconnection appeared to be the ideal observation time (37). Confirmation of both entrance and exit block with pulmonary vein isolation improves long-term success rates through more rigorous assessment of the encircling ablation lesion set (38). Another means to test the integrity of the ablation lines is to test for noncapture along the ablation lines with pacing. This approach resulted in significantly lower rates of AF recurrence in a prospective trial (39). Adenosine administration after initial isolation to examine for both pulmonary vein reconnection and spontaneous ectopy can be used to identify gaps in the ablation line or identify pulmonary veins at higher risk of recurrence (40). Finally, general anesthesia compared to conscious sedation lowers reconnection rates in those patients with recurrences that were restudied (19% vs. 42%, respectively) (41).
It must be emphasized that no systematic study has been performed to evaluate these approaches and technologies to determine their true impact on durable pulmonary vein isolation. As discussed previously, absence of AF recurrence does not necessarily indicate a higher rate of pulmonary vein isolation. Further, analysis of patients that present for repeat study can provide an understanding of the potential mechanisms responsible for pulmonary vein reconnection, however these patients are a biased population that have failed the initial procedure and as such may have unique anatomic or physiologic characteristics that impact pulmonary vein reconnection rates that may not translate entirely to a general ablation population.
Ablation of nonpulmonary vein triggers and arrhythmia maintenance mechanisms
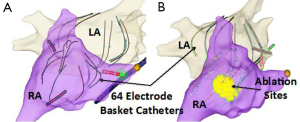
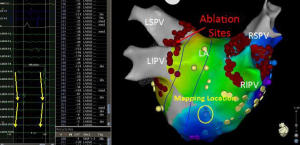
Based on the results of multiple studies it is clear that pulmonary vein triggers are not the only mechanisms responsible for AF. In patients with persistent and long-standing persistent AF it is generally recognized that pulmonary vein isolation may not be enough. This conclusion is largely derived by many studies that have shown that additional ablation beyond pulmonary vein isolation improves outcomes. Several approaches for directing additional ablation beyond pulmonary vein isolation have been shown to improve outcomes in patients with persistent AF. These ablation approaches include: atrial substrate modification (42), targeting complex fractionated electrograms (CFAE) (43), ligament of Marshall ablation (44), ablation of ganglion plexi (45), linear ablation (46), focal impulse or rotor modulation (FIRM) (47), and rapid drivers/dominant frequency (48). Often with more advanced subtypes of AF, if FIRM, CFAE, or rapid drivers are targeted a biatrial approach is needed. Figure 2 shows a patient that underwent FIRM guided ablation with biatrial contact mapping that resulted in the targeting of a right atrial inferolateral rotor.
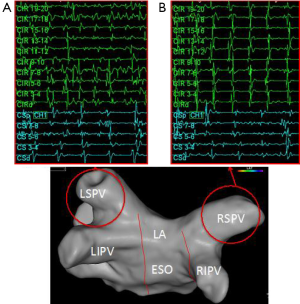
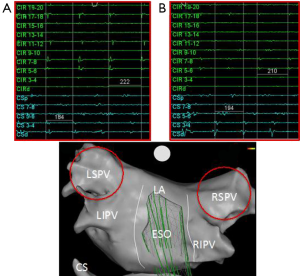
One of the interesting electrophysiology phenomena supporting the role of these other arrhythmia drivers is the cycle length of the pulmonary vein triggers compared to that found in the coronary sinus. Early in the disease process the electrogram frequency in one or more of the pulmonary veins is often much higher than that in the coronary sinus (Figures 1,3). In these patients it is easy to surmise that the pulmonary veins are drivers of the arrhythmia. Even in patients with early transition from paroxysmal to persistent AF a different pattern can often be seen where the pulmonary vein frequency is much lower than that observed in the coronary sinus (Figure 4). While collision of multiple slower pulmonary vein drivers is still a possibility, these findings also suggest additional rapid drivers or mechanisms of arrhythmia maintenance may be involved.
Since ablation of these extrapulmonary vein targets has shown benefit in more advanced subtypes of AF, the question really is if these approaches are helpful in early stages of AF or if targeting them can replace pulmonary vein isolation. The discrepancy between the clinical outcomes observed after AF ablation and actual durable pulmonary vein isolation suggests the possibility that ablation of one or more of the other arrhythmia triggers may be as important as the procedure intended to isolate the pulmonary vein triggers. One of the challenges in questioning the independent role of pulmonary vein isolation is that many of these other targets are often incidentally treated during pulmonary vein isolation, particularly when a wide area circumferential approach is utilized.
Ganglion plexi modification
It has been postulated that the autonomic nervous system (both sympathetic and parasympathetic) plays and important role in modulating AF triggers and substrate. For example, in a study of 242 paroxysmal AF patients, three groups were compared; (I) standard pulmonary vein isolation; (II) ablation of the main ganglion plexi of the left atrium; and (III) both pulmonary vein isolation and left atrial ganglion plexi ablation. Over a 2-year follow-up period freedom from AF or atrial tachycardia was achieved in 56%, 48%, and 74% of the patients, respectively (P=0.004) (49). Although there was augmented benefit with combining ablation with ganglion plexi modification, success rates without pulmonary vein isolation were worse than the standard approach. The synergy noted with ablation of both targets may be explained in a study of 63 patients with paroxysmal AF. Ganglion plexi ablation alone before pulmonary vein isolation significantly decreased the occurrence of pulmonary vein firing in 75% of patients and reduced the inducibility of sustained AF in 68% (50).
Dominant frequency/rapid atrial drivers
The importance of non-pulmonary vein triggers for AF treatment is becoming an increasingly focus of many studies. For instance, the RADAR-AF trial evaluated the utility of treating high-frequency sources compared with pulmonary vein isolation (51). The trial included both patients with paroxysmal (n=115) and persistent (n=117) patients. These patients were randomized 1:1 to high-frequency source ablation versus circumferential pulmonary vein isolation. In the paroxysmal cohort, the freedom from AF was 69% in the high frequency source ablation group vs. 69% in the pulmonary isolation group (P=0.04 for noninferiority). Less adverse events were noted in the high frequency source ablation group (9% vs. 24%). In the persistent group, the comparison was between pulmonary vein isolation with high frequency source ablation versus pulmonary vein isolation alone. Freedom from AF at 12 months was 52% vs. 39%, respectively (P for superiority =0.15). More adverse events were noted in the combination therapy group (24% vs. 10%). We have found that rapid drivers can be sorted by morphology and then ranked by frequency and activation patterns using standard mapping systems with multi-polar mapping catheters (Figure 5). We have not studied these mapping techniques in patients with paroxysmal AF to determine if they can augment or replace pulmonary vein isolation.
Ligament of Marshall/vein of Marshall/Marshall tract ganglion plexus
There are no trials that have looked at ablation of this region alone vs. pulmonary vein isolation. In this area there is a ganglion described as the Marshall tract ganglion plexus. This ganglion was 1 of 5 left atrial plexi targeted that significantly impacted pulmonary vein firing and inducibility of AF in the study described previously (50). There are data to suggest the role of this region in AF driven by high adrenergic states. In addition, recurrent induction of AF in a young patient with lone AF from the ligament of Marshall has been described (50). This is a challenging area to sort out independently for benefit with focused ablation from pulmonary vein isolation alone as many of the insertions of the ligament or vein of Marshall are along the anterior ridge between the left atrial appendage and left superior vena cava.
Focal impulse or rotor modulation (FIRM)
In the precise rotor elimination without concomitant pulmonary vein isolation for subsequent elimination (PRECISE) of paroxysmal AF trial 31 consecutive patients underwent FIRM without additional pulmonary vein isolation. In this study the investigators found stable rotors/focal sources in all patients with an average of 2.5±1.4 sources/patient. Of these sources, 66% were found in the left atrium and 34% in the right atrium. Ablation of these sites resulted in non-inducibility or an AF cycle lengthening of >10% in all patients. This focused ablation resulted in a 190 (IQR 117-334) day freedom from AF of 82.6%. In comparison to historical controls that received pulmonary vein isolation alone, this freedom from AF was significantly better (82.6% vs. 58.3%, respectively, P<0.05) (52). Outcomes with FIRM ablation alone without pulmonary vein isolation are anticipated soon.
Complex fractionated electrograms (CFAE)
In the selective complex fractionated atrial electrograms targeting for AF (SELECT AF) study trial patients with high burden paroxysmal AF were randomized to receive pulmonary vein isolation followed by ablation of either CFAE sites using an automated mapping system (confidence interval >7) vs. regions with continuous electrical activity (very high frequency). At 1-year follow-up, freedom from AF/atrial flutter/atrial tachycardia was significantly higher in the group with CFAE ablation versus the group with continuous electrogram ablation (50% vs. 28%; P=0.03) (53).
Summarizing these multiple studies of patients with paroxysmal AF, FIRM ablation or targeting of rapid stable atrial drivers appeared to show the most promise as stand alone therapies without pulmonary vein isolation; although randomized data is only available with targeting rapid atrial drivers (continuous atrial electrograms). Importantly, the incidental targeting of these rotors, focal impulses, or drivers may be the mechanism behind the low arrhythmia recurrence rates with pulmonary vein isolation procedures that often do not result in durable pulmonary vein isolation. It is unclear how often the rotors, impulses, or drivers stem from ganglion plexi, but targeting of these plexi sites alone appears to be insufficient. However ganglion plexi ablation as an adjuvant strategy to pulmonary vein isolation improves outcomes likely because of it affect on the autonomic modulation of arrhythmia triggers. Also, all of these methods require careful study, independent of their effects on AF, to understand long-term risks of atrial flutter, atrial tachycardia and ventricular arrhythmia.
Conclusions
Understanding the outcomes of catheter ablation of AF, in particular pulmonary vein isolation is severely limited by diverse ablation methodologies that do not seem to result in durable pulmonary vein isolation. Without durable pulmonary isolation ablation, it is unclear if ablation strategies need to be modified to include extrapulmonary vein ablation targets in combination with pulmonary vein isolation or alone to improve long-term procedural success rates. The marked discrepancy between AF ablation procedure success rates and actual long-term pulmonary vein isolation rates does suggest that targeting other mechanisms can be considered to achieve similar or better results when compared to pulmonary vein isolation alone. Of these approaches targeting rapid focal drivers or FIRM ablation have shown the most promise and may also provide mechanistic understanding behind why pulmonary vein isolation procedures succeed without durable pulmonary vein isolation.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Jaïs P, Cauchemez B, Macle L, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation 2008;118:2498-505. [PubMed]
- Packer DL, Kowal RC, Wheelan KR, et al. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol 2013;61:1713-23. [PubMed]
- Pappone C, Augello G, Sala S, et al. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. J Am Coll Cardiol 2006;48:2340-7. [PubMed]
- Stabile G, Bertaglia E, Senatore G, et al. Catheter ablation treatment in patients with drug-refractory atrial fibrillation: a prospective, multi-centre, randomized, controlled study (Catheter Ablation For The Cure Of Atrial Fibrillation Study). Eur Heart J 2006;27:216-21. [PubMed]
- Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA 2005;293:2634-40. [PubMed]
- Wilber DJ, Pappone C, Neuzil P, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA 2010;303:333-40. [PubMed]
- Cosedis Nielsen J, Johannessen A, Raatikainen P, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med 2012;367:1587-95. [PubMed]
- Morillo CA, Verma A, Connolly SJ, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): a randomized trial. JAMA 2014;311:692-700. [PubMed]
- Bunch TJ, May HT, Bair TL, et al. Increasing time between first diagnosis of atrial fibrillation and catheter ablation adversely affects long-term outcomes. Heart Rhythm 2013;10:1257-62. [PubMed]
- Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659-66. [PubMed]
- Chen SA, Hsieh MH, Tai CT, et al. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation 1999;100:1879-86. [PubMed]
- Jaïs P, Weerasooriya R, Shah DC, et al. Ablation therapy for atrial fibrillation (AF): past, present and future. Cardiovasc Res 2002;54:337-46. [PubMed]
- Oral H, Scharf C, Chugh A, et al. Catheter ablation for paroxysmal atrial fibrillation: segmental pulmonary vein ostial ablation versus left atrial ablation. Circulation 2003;108:2355-60. [PubMed]
- Zhang B, Zhen Y, Tao A, et al. Efficacy of selective arrhythmogenic pulmonary veins isolation versus empirical all pulmonary veins isolation for atrial fibrillation: a meta-analysis of randomized and observational studies. J Interv Card Electrophysiol 2014;39:233-40. [PubMed]
- Ouyang F, Tilz R, Chun J, et al. Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation 2010;122:2368-77. [PubMed]
- Sawhney N, Anousheh R, Chen WC, et al. Five-year outcomes after segmental pulmonary vein isolation for paroxysmal atrial fibrillation. Am J Cardiol 2009;104:366-72. [PubMed]
- Chao TF, Tsao HM, Lin YJ, et al. Clinical outcome of catheter ablation in patients with nonparoxysmal atrial fibrillation: results of 3-year follow-up. Circ Arrhythm Electrophysiol 2012;5:514-20. [PubMed]
- Callans DJ, Gerstenfeld EP, Dixit S, et al. Efficacy of repeat pulmonary vein isolation procedures in patients with recurrent atrial fibrillation. J Cardiovasc Electrophysiol 2004;15:1050-5. [PubMed]
- Verma A, Kilicaslan F, Pisano E, et al. Response of atrial fibrillation to pulmonary vein antrum isolation is directly related to resumption and delay of pulmonary vein conduction. Circulation 2005;112:627-35. [PubMed]
- McGann CJ, Kholmovski EG, Oakes RS, et al. New magnetic resonance imaging-based method for defining the extent of left atrial wall injury after the ablation of atrial fibrillation. J Am Coll Cardiol 2008;52:1263-71. [PubMed]
- Ranjan R, Kato R, Zviman MM, et al. Gaps in the ablation line as a potential cause of recovery from electrical isolation and their visualization using MRI. Circ Arrhythm Electrophysiol 2011;4:279-86. [PubMed]
- Kowalski M, Grimes MM, Perez FJ, et al. Histopathologic characterization of chronic radiofrequency ablation lesions for pulmonary vein isolation. J Am Coll Cardiol 2012;59:930-8. [PubMed]
- Malasana G, Day JD, Weiss JP, et al. A strategy of rapid cardioversion minimizes the significance of early recurrent atrial tachyarrhythmias after ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2011;22:761-6. [PubMed]
- Deftereos S, Giannopoulos G, Efremidis M, et al. Colchicine for prevention of atrial fibrillation recurrence after pulmonary vein isolation: mid-term efficacy and effect on quality of life. Heart Rhythm 2014;11:620-8. [PubMed]
- Deftereos S, Giannopoulos G, Kossyvakis C, et al. Colchicine for prevention of early atrial fibrillation recurrence after pulmonary vein isolation: a randomized controlled study. J Am Coll Cardiol 2012;60:1790-6. [PubMed]
- Caplice NM, Bunch TJ, Stalboerger PG, et al. Smooth muscle cells in human coronary atherosclerosis can originate from cells administered at marrow transplantation. Proc Natl Acad Sci U S A 2003;100:4754-9. [PubMed]
- Matsuo S, Yamane T, Tokuda M, et al. The dormant epicardial reconnection of pulmonary vein: an unusual cause of recurrent atrial fibrillation after pulmonary vein isolation. Pacing Clin Electrophysiol 2008;31:920-4. [PubMed]
- Tan AY, Chou CC, Zhou S, et al. Electrical connections between left superior pulmonary vein, left atrium, and ligament of Marshall: implications for mechanisms of atrial fibrillation. Am J Physiol Heart Circ Physiol 2006;290:H312-22. [PubMed]
- Jiang RH, Po SS, Tung R, et al. Incidence of pulmonary vein conduction recovery in patients without clinical recurrence after ablation of paroxysmal atrial fibrillation: mechanistic implications. Heart Rhythm 2014;11:969-76. [PubMed]
- Parmar BR, Jarrett TR, Burgon NS, et al. Comparison of left atrial area marked ablated in electroanatomical maps with scar in MRI. J Cardiovasc Electrophysiol 2014;25:457-63. [PubMed]
- McGann C, Akoum N, Patel A, et al. Atrial fibrillation ablation outcome is predicted by left atrial remodeling on MRI. Circ Arrhythm Electrophysiol 2014;7:23-30. [PubMed]
- Cappato R, Negroni S, Pecora D, et al. Prospective assessment of late conduction recurrence across radiofrequency lesions producing electrical disconnection at the pulmonary vein ostium in patients with atrial fibrillation. Circulation 2003;108:1599-604. [PubMed]
- Ouyang F, Antz M, Ernst S, et al. Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from double Lasso technique. Circulation 2005;111:127-35. [PubMed]
- Sciarra L, Golia P, Natalizia A, et al. Which is the best catheter to perform atrial fibrillation ablation? A comparison between standard ThermoCool, SmartTouch, and Surround Flow catheters. J Interv Card Electrophysiol 2014;39:193-200. [PubMed]
- Park CI, Lehrmann H, Keyl C, et al. Mechanisms of pulmonary vein reconnection after radiofrequency ablation of atrial fibrillation: the deterministic role of contact force and interlesion distance. J Cardiovasc Electrophysiol 2014;25:701-8. [PubMed]
- Kühne M, Suter Y, Altmann D, et al. Cryoballoon versus radiofrequency catheter ablation of paroxysmal atrial fibrillation: biomarkers of myocardial injury, recurrence rates, and pulmonary vein reconnection patterns. Heart Rhythm 2010;7:1770-6. [PubMed]
- Nakamura K, Naito S, Kaseno K, et al. Optimal observation time after completion of circumferential pulmonary vein isolation for atrial fibrillation to prevent chronic pulmonary vein reconnections. Int J Cardiol 2013;168:5300-10. [PubMed]
- Chen S, Meng W, Sheng He D, et al. Blocking the pulmonary vein to left atrium conduction in addition to the entrance block enhances clinical efficacy in atrial fibrillation ablation. Pacing Clin Electrophysiol 2012;35:524-31. [PubMed]
- Steven D, Sultan A, Reddy V, et al. Benefit of pulmonary vein isolation guided by loss of pace capture on the ablation line: results from a prospective 2-center randomized trial. J Am Coll Cardiol 2013;62:44-50. [PubMed]
- Cheung JW, Lin FS, Ip JE, et al. Adenosine-induced pulmonary vein ectopy as a predictor of recurrent atrial fibrillation after pulmonary vein isolation. Circ Arrhythm Electrophysiol 2013;6:1066-73. [PubMed]
- Di Biase L, Conti S, Mohanty P, et al. General anesthesia reduces the prevalence of pulmonary vein reconnection during repeat ablation when compared with conscious sedation: results from a randomized study. Heart Rhythm 2011;8:368-72. [PubMed]
- Lin YJ, Chang SL, Lo LW, et al. A prospective and randomized comparison of limited versus extensive atrial substrate modification after circumferential pulmonary vein isolation in nonparoxysmal atrial fibrillation. J Cardiovasc Electrophysiol 2014;25:803-12. [PubMed]
- Nademanee K, McKenzie J, Kosar E, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol 2004;43:2044-53. [PubMed]
- Hwang C, Chen PS. Ligament of Marshall: why it is important for atrial fibrillation ablation. Heart Rhythm 2009;6:S35-40. [PubMed]
- Pokushalov E, Romanov A, Artyomenko S, et al. Ganglionated plexi ablation for longstanding persistent atrial fibrillation. Europace 2010;12:342-6. [PubMed]
- Knecht S, Hocini M, Wright M, et al. Left atrial linear lesions are required for successful treatment of persistent atrial fibrillation. Eur Heart J 2008;29:2359-66. [PubMed]
- Narayan SM, Krummen DE, Shivkumar K, et al. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol 2012;60:628-36. [PubMed]
- Kumagai K, Sakamoto T, Nakamura K, et al. Combined dominant frequency and complex fractionated atrial electrogram ablation after circumferential pulmonary vein isolation of atrial fibrillation. J Cardiovasc Electrophysiol 2013;24:975-83. [PubMed]
- Katritsis DG, Pokushalov E, Romanov A, et al. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: a randomized clinical trial. J Am Coll Cardiol 2013;62:2318-25. [PubMed]
- Nakagawa H, Scherlag BJ, Patterson E, et al. Pathophysiologic basis of autonomic ganglionated plexus ablation in patients with atrial fibrillation. Heart Rhythm 2009;6:S26-34. [PubMed]
- Atienza F, Almendral J, Ormaetxe JM, et al. Comparison of Radiofrequency Catheter Ablation of Drivers and Circumferential Pulmonary Vein Isolation in Atrial Fibrillation: A Noninferiority Randomized Multicenter RADAR-AF Trial. J Am Coll Cardiol 2014;64:2455-67. [PubMed]
- Narayan SM, Krummen DE, Donsky A, et al. Treatment Of Paroxysmal Atrial Fibrillation By argeted Elimination Of Stable Rotors And Focal Sources Without Pulmonary Vein Isolation: Te Precise Rotor Elimination Without Concomitant Pulmonary Vein Isolation For Subsequent Elimination Of PAF (PRECISE). Hear Rhythm 2013;LB01-05.
- Verma A, Sanders P, Champagne J, et al. Selective complex fractionated atrial electrograms targeting for atrial fibrillation study (SELECT AF): a multicenter, randomized trial. Circ Arrhythm Electrophysiol 2014;7:55-62. [PubMed]


