Thoracocentesis: from bench to bed
Introduction
Thoracocentesis (from the Greek words, thorax + centesis, puncture) is an invasive procedure associated with removal of fluid or air from the pleural space for diagnostic or therapeutic purposes (1).
This can be performed by inserting carefully a needle into the pleural space in order to aspirate the pathologically collected fluid or air and allow the compressed lung to re-inflate. Ultrasound guided needle aspiration is a very useful technique and, whenever is possible, should be performed in order to reduce complications (2,3).
Indications
- Diagnostic purposes (fluid analysis);
- Therapeutic purposes (removal of fluid/air from the thoracic cavity in order to improve patient comfort and lung function).
The most common causes of pleural effusions are cancer, congestive heart failure, pneumonia, tuberculosis and recent surgery.
Contraindications
Contraindications to thoracocentecis are an uncooperative patient, severe hemostasis alteration, hemodynamic instability, severe respiratory failure or a pleural effusion considered too small to be safely taped (4).
Relative contraindications include cases in which the site of insertion has known bullous disease (e.g., emphysema) and the use of positive end-expiratory pressure (PEEP).
The aspiration preferably should not exceed 1,5 Lt as there is a potential low risk of development of re-expansion pulmonary edema.
Complications
Potential complications of thoracentesis include pain, pneumothorax, shortness of breath, cough, and vasovagal reaction.
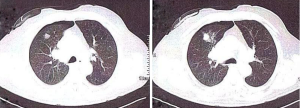
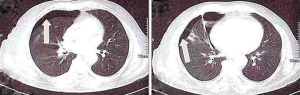
Other less common complications of thoracentesis include re-expansion pulmonary edema, inadvertent liver or splenic puncture, bleeding from a lacerated intercostal vessel, induction of a pleural infection, subcutaneous emphysema, air embolism, a sheared-off catheter in the pleural space, as well as subcutaneous hematoma (5) (Figures 1,2).
Procedure-technique-equipment-position
The procedure is performed by an experienced interventional radiologist or a well-trained physician with the patient in a seated position (unless the clinical situation does not allow) (2,3).
Ultrasound-guided thoracocentecis is a valuable technique that could be performed in order to reduce complications especially in patients who are on mechanical ventilator support (iatrogenic pneumothorax in a patient on positive-pressure mechanical ventilatory support is particularly dangerous because there is a high risk of tension pneumothorax) or in case of a small effusion (3).
The recommended location for the needle insertion varies depending upon the source. Some sources recommend the mid-axillary line in the 6th, 7th, or 8th intercostal space. It is critical that the patient hold his or her breath to avoid piercing of the lung.
The pleural fluid collection is located and marked under real-time ultrasound guidance. Multiple scans are obtained in transverse, oblique, and sagittal planes.
After preparation of the site, needle insertion is performed at the marked point in the middle of the appropriate intercostal space, with the insertion assembly held at the same angle as was the probe, taking into account to avoid the blood vessels and nerves that run down the caudal edge of each rib.
The procedure must be performed under aseptic conditions using: (I) local anesthetic, sterile needle; (II) butterfly; (III) a plastic flexible catheter-needle system; (IV) three-way; (V) syringe. Lung sliding should be assessed before and following thoracentesis. The loss of lung sliding following thoracentesis when it was present before the procedure indicates that there has been a procedure-related pneumothorax. The finding of lung sliding following thoracentesis obviates the need for chest radiography performed in order to rule out pneumothorax (3,6). There is a suggestion that drainage of large amounts of fluid at ultrasound-guided thoracentesis is a risk factor for pneumothorax (7).
Fluid analysis
The collected fluid should differentiate from transudate or exudate.
A transudative effusion is caused by increased hydrostatic forces. The permeability of the capillaries to proteins is normal. An exudative effusion is caused by increased capillary permeability or lymphatic obstruction.
According to Light’s criteria (8) the pleural fluid is an exudate if one or more of the following criteria are met:
- Pleural fluid protein divided by serum protein >0.5;
- Pleural fluid LDH divided by serum LDH >0.6;
- Pleural fluid LDH more than 2/3 the upper limits of normal serum LDH
Exudate fluid etiology (9)
Common causes
- Malignancy
- Parapneumonic effusions
- Tuberculosis
Less common causes
- Pulmonary embolism
- Rheumatoid arthritis
- Autoimmune diseases
- Benign asbestos effusion
- Pancreatitis
- Post-myocardial infarction syndrome
- Post-coronary artery bypass graft
Rare causes
- Yellow nail syndrome
- Drugs
- Fungal infections
Transudate fluid etiology (9)
Very common causes
- Left ventricular failure
- Liver cirrhosis
Less common causes
- Hypoalbuminaemia
- Peritoneal dialysis
- Hypothyroidism
- Nephrotic syndrome
- Mitral stenosis
Rare causes
- Constrictive pericarditis
- Urinothorax
- Superior vena cava obstruction
- Meigs’ syndrome
Appearance
The appearance of the pleural fluid and any odour should be recorded. A pleural fluid haematocrit is helpful in the diagnosis of haemothorax (9).
pH
A pH in a parapneumonic effusion less than 7.20 suggests the need for drainage of the fluid (empyema), usually with a chest tube. It has been suggested that a pH in this range correlates with a life expectancy of around 30 days (10).
Glucose
Low pleural fluid glucose level (<3.4 mmol/L) suggests a complicated parapneumonic or malignant effusion or effusion due to empyema, rheumatoid disease, lupus, tuberculosis or esophageal rupture (9).
Cytology
Cytology is an important tool in identifying effusions due to malignancy. Immunocytochemistry should be used to differentiate between malignant cell types and can be very important in guiding oncological therapy (9).
Other markers are N-terminal pro-brain natriuretic peptide (NT-proBNP), which is a sensitive marker of both systolic and diastolic cardiac failure, amylase, in suspected cases of esophageal rupture or effusions associated with pancreatic diseases, and tumor markers. Cultures are necessary in the differential diagnosis of infections.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Williamson JA. Construct validation of a small-animal thoracocentesis simulator. J Vet Med Educ 2014;41:384-9. [PubMed]
- Rajesh G. Ultrasound Guided Procedures in Emergency Medicine Practice – Thoracentesis. Ultrasound Guide for Emergency Physicians 2008. Available online: http://www.sonoguide.com/thoracentesis.html
- Mayo PH, Doelken P. Pleural ultrasonography. Clin Chest Med 2006;27:215-27. [PubMed]
- Fartoukh M, Azoulay E, Galliot R, et al. Clinically documented pleural effusions in medical ICU patients: how useful is routine thoracentesis? Chest 2002;121:178-84. [PubMed]
- Jones PW, Moyers JP, Rogers JT, et al. Ultrasound-guided thoracentesis: is it a safer method? Chest 2003;123:418-23. [PubMed]
- Koenig SJ, Narasimhan M, Mayo PH. Thoracic ultrasonography for the pulmonary specialist. Chest 2011;140:1332-41. [PubMed]
- Josephson T, Nordenskjold CA, Larsson J, et al. Amount drained at ultrasound-guided thoracentesis and risk of pneumothorax. Acta Radiol 2009;50:42-7. [PubMed]
- Light RW. The undiagnosed pleural effusion. Clin Chest Med 2006;27:309-19. [PubMed]
- Hooper C, Lee YC, Maskell N, et al. Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii4-17. [PubMed]
- Light RW. Clinical practice. Pleural effusion. N Engl J Med 2002;346:1971-7. [PubMed]