
Preoperative predictors of lymph node metastasis in clinical T1 adenocarcinoma
Introduction
In recent years, detection of smaller pulmonary nodules, became possible with the development of computed tomography (CT) and the spread of high-resolution (HR) CT as well as low-dose CT examinations.
Some reports have stated that sublobar resection, including segmentectomy or wedge resection, should be performed for small nodules. Clinical studies demonstrated efficacy (1-4). Sublobar resection is more useful, particularly in patients who have many complications, low respiratory function, or advanced aged (5,6). However, higher local recurrence after sublobar resection was observed when a negative surgical margin had been confirmed pathologically (7). The efficacy of sublobar resection has been prospectively evaluated for small nodules (8,9). The appropriate choice is important when considering sublobar resection, based on whether the lymph node is negative for metastasis (10).
Lymph node metastasis can be predicted by CT and 18F-fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT); nevertheless, the accuracy is not high. For this reason, many studies have tried to identify predictors for lymph node metastasis.
The proposed predictors of lymph node metastasis have been cancer embryonic antigen (CEA) (11-20), maximum standardized uptake (SUVmax) (19,21-23), the size of tumor (10,11,18,24-26), and solid component of tumor (18,20,22,25,26,33); most of these studies were conducted based on the Tumor Node Metastasis (TNM) classification, 7th edition (27).
The TNM classification was updated to the eighth edition (28) in January 2017, and the new subcategory “the solid component of tumor” was added to the new criteria of the tumor category (T). There remain many unclear features regarding predictors of lymph node metastasis among the patients with T1 adenocarcinoma based on the TNM classification, 8th edition. Therefore, the aim of this study was to identify the preoperative predictors of lymph node metastasis in clinical T1 adenocarcinoma by comparing clinicopathological characteristics between the groups with and without lymph node metastasis.
Methods
Study design, patients, and approval
We performed a retrospective observational single-center study of patients with primary lung adenocarcinoma and conducted at the Sendai Kousei Hospital, Miyagi, Japan. All patients gave written informed consent. From January 2012 to September 2019, we included 515 patients who underwent curative lobectomy or segmentectomy and mediastinal lymph node dissection among clinical T1 adenocarcinoma based on the Union for International Cancer Control (UICC)-TNM staging 8th edition.
The protocols of data collection and analysis were approved by our institutional review board (IRB No. 1-23) at Sendai Kousei Hospital; the requirement for written informed consent was waived because the data were analyzed retrospectively. This article was based on the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement: guidelines for reporting observational studies (29).
The patients were divided into a group with lymph node metastasis (positive group) and a group without lymph node metastasis (negative group). We retrospectively analyzed and compared the following clinicopathological factors between the two groups: tumor markers, maximum standardized uptake value in FDG-PET/CT, and preoperative tumor size.
The exclusion criteria were follows: (I) multiple lesions; (II) induction therapy; (III) preoperative identification of mediastinal lymph node metastases using endobronchial ultrasound-guided transbronchial needLe aspiration (EBUS-TBNA); (IV) mediastinal lymph nodes for which the minor axis was 10 mm or more in size; and (V) pathologically diagnosed lung metastasis from lung cancer (a second primary lung cancer was not excluded).
Radiological measurements and clinical diagnosis
Evaluation of CT findings
All patients underwent preoperative thin-slice contrast-enhanced CT of the 1–2-mm slice 1 month before lobectomy at Sendai Kousei Hospital. We used 320-row-detector (area detector) CT scanners (Aquilion 64, Toshiba Medical Systems, Otawara, Tochigi, Japan) to acquire chest images using the following settings: 1.0-mm section width with 1.0-mm reconstruction interval, volume scan, tube voltage 120 kVp, 100 mA, 512×512-pixel resolution, 0.35-second/lot scanning time, a high-spatial reconstruction algorithm with a 35-cm field of view.
The mediastinal window had a window level 10 Hounsfield units (HU) and window width 300 HU. The lung window had a window level –700 HU and window width –1,500 HU.
The total tumor diameter (TTD) expressed the whole tumor diameter including the GGN lesion, and the consolidation diameter (CD) was solid component diameter of the tumor. The consolidation tumor ratio (CTR) was defined as the ratio of the CD to TTD.
At least two surgeons evaluated radiation tumor findings using thin section CT and recorded clinical TNM stage according to the 8th edition criteria of the TNM classification. All tumor findings were reevaluated according to the TNM 8th edition following the scoring according to the TNM 7th edition.
Evaluation of 18F-fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) findings
The data of FDG-PET/CT were collected in 470 (91.2%) patients, and FDG-PET/CT scanning was performed according to the procedures of our hospital (Sendai Kousei Hospital, Miyagi, Japan). Patients stopped consuming sugar within at least 4 hours before a scan. Blood sugar levels were measured before starting the examination, and the scan was postponed when it was 200 mg/dL or more. First, patients were intravenously administered 5.735 MBq/kg 18F-FDG, and then rested for 90 minutes. We identified the position of the lesion using PET. Using a low-dose of thickness of 3.75 mm from the base of the skull of each patient to the femoral center, the decay correction of the non-revision CT image was subsequently obtained using a standard protocol. These PET scanning/images were acquired at bed positions of 7–9 in a Discovery ST elite PET/CT scanner (GE Medical Systems, Waukesha, WI, USA). The raw PET data were rebuilt using a section image of thickness 3.27 mm to evaluate volume changes in the 3D-ordered subsets expectation maximization (OSEM) algorithm that incorporated CT-based decay correction (2 iteration/28 subsets).
We used a workstation (Advantage Workstation 4.2) for indication and image analysis and calculated SUVmax of the primary tumor. All PET/CT images were interpreted by an experienced nuclear radiation engineer. The definitions of nodal station were based on the International Association of the Study of Lung Cancer (IASLC) lymph node map (30) and pathological diagnoses were based on the 2011 IASLC classification (31).
Preoperative diagnosis
In this study, in most cases, bronchoscopy was performed to confirm a diagnosis preoperatively. When a diagnosis was not confirmed by bronchoscopy, CT-guided needle lung biopsy was performed in several cases.
In cases that did not acquire a diagnosis after these examinations, for example, in particular, a peripheral small nodule, if the patient agreed to undergo lung resection after we provided sufficient explanation for its requirement, lung resection was planned.
Surgical procedures
Surgery was performed under general anesthesia and single-lung ventilation in the lateral position. Thoracotomy was performed by posterolateral incision, and video-assisted thoracic surgery (VATS) was performed using three port incisions. For all patients, segmentectomy or lobectomy was performed. When a diagnosis was not obtained, we confirmed the diagnosis by needle lung (tumor) biopsy or wedge lung (tumor) resection. If it was primary lung cancer, we chose lobectomy. When the invasive diameter of a tumor was 5 mm or less, sublobar resection was performed in patients who could not tolerate lobectomy.
The pulmonary vein, pulmonary artery, bronchus, and lung parenchyma were divided using a stapler (Endo GIATM, Covidien, USA, or ECHELON FLEX TM, ETHICON, USA) or an energy device (HARMONIC TM, ETHICON, US, or THUNDERBEAT TM, OLYMPUS, Japan) or ligated, depending on the situation. Mediastinal lymph node dissection was performed in most patients, but was not performed in patients 80 years or older and in those with severe cardiovascular complications. Lymph node dissection was performed using a protocol modified to the recommendation of European Society of Thoracic Surgeons (ESTS) (32). In our hospital, we perform mediastinal lymph node dissection (ND2a-1). When we perform lung resection for patients suspected having mediastinal lymph node metastasis, we dissect the upper, middle, and lower mediastinal lymph nodes (ND2a-2) (Table 1).

Full table
Data collection
In our institution, laboratory tests, CT, PET/CT, and magnetic resonance imaging (MRI) are performed preoperatively. The following data were collected: age, gender, Brinkman index, clinical stage, CEA, cytokeratin 19 fragments (CYFRA), TTD, CD, CTR, SUVmax, type of surgical approach, surgical procedure, tumor location, extent of lymph node dissection, histological subtype, and pathological involvement.
Statistical analysis
The Student’s t-test, and Mann-Whitney U test were used to compare continuous variables and the Chi-square test was used to compare categorical variables between the groups.
Receiver operating characteristic (ROC) curves to predict lymph node metastasis were used to determine the cutoff value that yielded optimal sensitivity and specificity using the Youden index for each variable.
The method using the Youden index was used to define the maximum potential effective cut-off value in the ROC curve and calculated (sensitivity + specificity − 1; Youden index), and the cut-point that acquired the maximum value was defined as the optimal cut-off value.
Univariate and multivariate analyses were performed to identify the predictors for lymph node involvement using logistic regression. All analyses were performed using JMP® 13 software (SAS Institute Inc., Cary, NC, USA). P values <0.05 were considered statistically significant.
Results
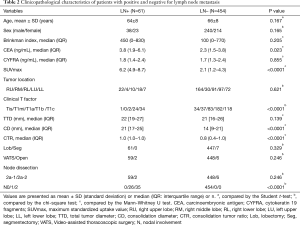
The mean age was 66 years (range, 40–86 years), and the details of all patients are shown in Table 2. The median value of CEA and SUVmax was significantly higher in the lymph node metastasis positive group (3.8 and 6.2) than in the negative group (2.3 and 2.1) (P=0.023 and P<0.0001). The clinical T factor showed one patient with Tis, two patients with T1a, 24 patients with T1b, and 34 patients with T1c in the lymph node metastasis positive group. There were 164 patients with Tis, 30 patients with T1mi, 91 patients with T1a, 97 patients with T1b, and 72 patients with T1c in the lymph node metastasis negative group. The median CD and CTR were significantly high in lymph node metastasis positive group compared to the negative group (P<0.0001 and <0.0001, respectively).
Full table
Regarding intraoperative characteristics, lobectomy was performed in 508 patients, and segmentectomy in eight patients, while there were 507 cases of VATS and eight cases of open chest surgery. Lymph node metastasis was present in 61 patients, including 26 (43%) with pN1 and 35 (57%) with pN2. The optimal cut-off values of age, Brinkman index, SUVmax, and CTR, defined by the Youden index to predict lymph node metastasis, were 72 [accuracy =0.33; sensitivity =0.89; specificity =0.26; area under the curve (AUC) =0.56; P=0.17], 390 (accuracy =0.58; sensitivity =0.54; specificity =0.59; AUC =0.56; P=0.21), 3.5 (accuracy =0.72; sensitivity =0.88; specificity =0.69; AUC =0.83; P<0.0001), and 0.85 (accuracy =0.58; sensitivity =0.85, specificity =0.54; AUC =0.72; P<0.0001) by the ROC curve.
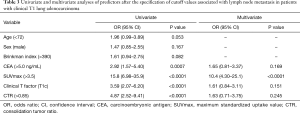
Univariate and multivariate analyses were performed to identify the predictors of lymph node metastasis between the two groups (Table 3). According to univariate analysis, CEA (>5.0 ng/mL) (P=0.0007), SUVmax (>3.5) (P<0.0001), clinical T factor (T1c) (P<0.0001), and CTR (>0.85) (P<0.0001) were significant predictors of lymph node metastasis. Multivariate analysis revealed SUVmax (>3.5) as independently associated with lymph node metastasis [odds ratio (OR) =10.4, P<0.0001].
Full table
Subgroup analysis
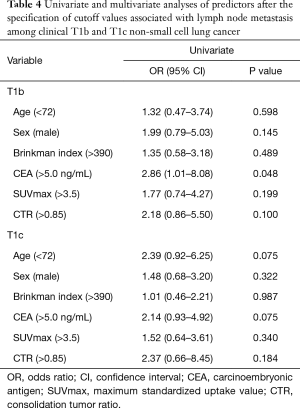
Among the patients with clinical T1mi, there were no patients with lymph node metastasis. Among the patients with clinical Tis and T1a, there were one and two patients with lymph node metastasis, respectively; however, the data could not be analyzed statistically. Among patients of cT1b, according to univariate analysis, CEA (>5.0) (P=0.048) was a significant predictor of lymph node metastasis. Among patients with cT1c, univariate analysis revealed no predictors of lymph node metastasis (Table 4).
Full table
Discussion
We found that SUVmax of a tumor (>3.5) in FDG-PET/CT predicted lymph node metastasis in clinical T1 lung adenocarcinoma. The value of CEA predicted lymph node metastasis in the subgroup analysis in clinical T1b. This study was a valuable report because there have been few reports regarding the predictors of lymph node metastasis particularly based on the TNM classification 8th edition and there have been few analyses of groups stratified as T1b or T1c.
Uptake of FDG correlated with the proliferative activity of tumor that independently became a prognostic factor for patients with lung cancer (33,34). We believe we can identify lymph node metastasis by CT and PET/CT. However, the sensitivity for node-positivity on CT is 50–80%, and the specificity is 50–90% (35,36). Authors also reported that 10 mm or less in the minor axis of lymph nodes indicated metastasis-positivity on CT. When accumulation of FDG in the lymph nodes was beyond 2.5, lymph node metastases were considered positive; nevertheless, lymph node metastases were often pathologically negative in such cases.
Sensitivity of clinical N using the PET was 60–90%, and the specificity was 70–100% (35,36). In another study, the uptake of FDG was shown to be a potential predictor of nodal metastasis in small primary NSCLC (37). Maeda et al. (21) reported that lymph node metastasis was not identified among patients with stage IA NSCLC when the value of SUVmax was 2 or less. In the present study, we also found no patients with lymph node metastasis when the value of SUVmax was 2 or less. Many studies found that high preoperative values of CEA were poor prognostic factors in stage I NSCLC (38).
CEA has been shown to predict lymph node metastasis (11-20). In the present study, among patients with clinical T1b, high CEA levels predicted lymph node metastasis on subgroup analysis. Some studies reported that there was no lymph node metastasis among the patients with GGNs (25,39,40). In fact, in stage IA NSCLC, lymph node metastasis was about 7–26% (19,41). Among the sub-centimeter NSCLC, Casiraghi et al. (42) reported no patients with lymph node metastasis, whereas Watanabe et al. (43) and Veronesi et al. (44) reported patients with lymph node metastasis. In the present study, there were no lymph node metastases in sub-centimeter tumors; however, small tumor size alone cannot be a reason for omitting lymph node dissection.
Incomplete dissection or sampling of lymph nodes could result in local recurrence. Many surgeons advocate for routine systemic nodal dissection so as to secure complete local control of an NSCLC, even if a patient’s disease is classified as clinical stage IA; this is because even small NSCLC lesions have considerable potential for lymph node metastasis (1).
Allen et al. (45) reported that mediastinal lymph node dissection or sampling causes a high rate of complications (about 38%) and omitting node dissection reduces complications and invasiveness and improves postoperative recovery. Systemic lymph node dissection could be avoided in selected patients if there appears to be no lymph node metastasis; that is, if we could identify predictors associated with pathological N0. If the minor axis of the mediastinal lymph node is less than 10 mm, it has been suggested that this predicts negative lymph node metastasis. Tsutani et al. (22) proposed “N0 criteria” using CT and PET/CT among the stage 1A adenocarcinoma, with a solid component diameter of <0.8 cm or SUVmax of <1.5 for selecting candidates for sublobar resection. Nevertheless, the definitive or universal criteria for prediction of lymph node metastasis have yet not been established.
Many studies have attempted to predict those patients who could avoid systemic lymph node dissection (43,46). We similarly intended to identify predictors of lymph node metastasis. In patients with adenocarcinoma, predictors of lymph node metastasis other than SUVmax in PET/CT were suggested as the following: CEA (12,14,19), solid component (22), GGO status (14,19), histological subtype (19). Our data suggest that in patients in whom accumulation of FDG in lung nodules is high, there are numerous lymph node metastases.
We need to mention some limitations to the present study. First, this was not a randomized controlled study. This study was a retrospective observational study in a single institution; therefore, the evidence level fell moderately. Second, measurements of tumors varied to some extent for each doctor. It appeared to improve when at least two or more doctors perform the measurement. Third, during the early period in this study, there were a few cases in which PET/CT was not performed for small tumors (mainly those <1 cm), which might have affected the results, whereas among patients for whom PET/CT was performed, SUVmax might depend on the modality.
In conclusion, our findings suggest that SUVmax and CEA would be useful as preoperative predictors of lymph node metastasis in patients of clinical T1 adenocarcinoma, with clinical T1b stratified, based on the TNM classification 8th edition. Further accumulation of data is needed to identify the predictors of lymph node metastasis among the patients with clinical T1 adenocarcinoma.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.74). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board of Sendai Kousei Hospital (IRB No. 1-23).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Carr SR, Schuchert MJ, Pennathur A, et al. Impact of tumor size on outcomes after anatomic lung resection for stage 1A non-small cell lung cancer based on the current staging system. J Thorac Cardiovasc Surg 2012;143:390-7. [Crossref] [PubMed]
- Martin-Ucar AE, Nakas A, Pilling JE, et al. A case-matched study of anatomical segmentectomy versus lobectomy for stage I lung cancer in high-risk patients. Eur J Cardiothorac Surg 2005;27:675-9. [Crossref] [PubMed]
- Schuchert MJ, Pettiford BL, Pennathur A, et al. Anatomic segmentectomy for stage I non-small-cell lung cancer: Comparison of video-assisted thoracic surgery versus open approach. J Thorac Cardiovasc Surg 2009;138:1318-25.e1. [Crossref] [PubMed]
- El-Sherif A, Gooding WE, Santos R, et al. Outcomes of sublobar resection versus lobectomy for stage I non-small cell lung cancer: a 13-year analysis. Ann Thorac Surg 2006;82:408-15; discussion 415-6. [Crossref] [PubMed]
- Tsubota N, Ayabe K, Doi O, et al. Ongoing prospective study of segmentectomy for small lung tumors. Study Group of Extended Segmentectomy for Small Lung Tumor. Ann Thorac Surg 1998;66:1787-90. [Crossref] [PubMed]
- Okada M, Nishio W, Sakamoto T, et al. Effect of tumor size on prognosis in patients with non-small cell lung cancer: the role of segmentectomy as a type of lesser resection. J Thorac Cardiovasc Surg 2005;129:87-93. [Crossref] [PubMed]
- Ichinose Y, Yano T, Yokoyama H, et al. The correlation between tumor size and lymphatic vessel invasion in resected peripheral stage I non-small-cell lung cancer. A potential risk of limited resection. J Thorac Cardiovasc Surg 1994;108:684-6. [Crossref] [PubMed]
- Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. [Crossref] [PubMed]
- ClinicalTrials.gov. Bethesda, MD: National Institutes of Health; Comparison of different types of surgery in treating patients with stage IA non-small cell lung cancer. Available at: http://clinicaltrials.gov/ct/show/NCT00499330. Accessed January 9, 2012.
- Bao F, Yuan P, Yuan X. Predictive risk factors for lymph node metastasis in patients with small size non-small cell lung cancer. J Thorac Dis 2014;6:1697-703. [PubMed]
- Takamochi K, Nagai K, Suzuki K, et al. Clinical predictors of N2 disease in non-small cell lung cancer. Chest 2000;117:1577-82. [Crossref] [PubMed]
- Takamochi K, Nagai K, Yoshida J, et al. Pathologic N0 status in pulmonary adenocarcinoma is predictable by combining serum carcinoembryonic antigen level and computed tomographic findings. J Thorac Cardiovasc Surg 2001;122:325-30. [Crossref] [PubMed]
- Sawabata N, Ohta M, Takeda S, et al. Serum carcinoembryonic antigen level in surgically resected clinical stage I patients with non-small cell lung cancer. Ann Thorac Surg 2002;74:174-9. [Crossref] [PubMed]
- Suzuki K, Kusumoto M, Watanabe S, et al. Radiologic classification of small adenocarcinoma of the lung: radiologic-pathologic correlation and its prognostic impact. Ann Thorac Surg 2006;81:413-9. [Crossref] [PubMed]
- Yoshino I, Ichinose Y, Nagashima A, et al. Kyushu Lung Cancer Surgery Cooperative Group. Clinical characterization of node-negative lung adenocarcinoma: results of a prospective investigation. J Thorac Oncol 2006;1:825-31. [Crossref] [PubMed]
- Inoue M, Minami M, Shiono H, et al. Clinicopathologic study of resected, peripheral, small-sized, non-small cell lung cancer tumors of 2 cm or less in diameter: Pleural invasion and increase of serum carcinoembryonic antigen level as predictors of nodal involvement. J Thorac Cardiovasc Surg 2006;131:988-93. [Crossref] [PubMed]
- Hattori A, Suzuki K, Matsunaga T, et al. Is limited resection appropriate for radiologically”solid” tumors in small lung cancers? Ann Thorac Surg 2012;94:212-5. [Crossref] [PubMed]
- Koike T, Koike T, Yamato Y, et al. Predictive risk factors for mediastinal lymph node metastasis in clinical stage IA non-small-cell lung cancer patients. J Thorac Oncol 2012;7:1246-51. [Crossref] [PubMed]
- Ye B, Cheng M, Li W, et al. Predictive factors for lymph node metastasis in clinical stage IA lung adenocarcinoma. Ann Thorac Surg 2014;98:217-23. [Crossref] [PubMed]
- Yu X, Li Y, Shi C, et al. Risk factors of lymph node metastasis in patients with non-small cell lung cancer ≤ 2 cm in size: A monocentric population-based analysis. Thorac Cancer 2018;9:3-9. [Crossref] [PubMed]
- Maeda R, Isowa N, Onuma H, et al. The maximum standardized 18F-fluorodeoxyglucose uptake on positron emission tomography predicts lymph node metastasis and invasiveness in clinical stage IA non-small cell lung cancer. Interact Cardiovasc Thorac Surg 2009;9:79-82. [Crossref] [PubMed]
- Tsutani Y, Miyata Y, Nakayama H, et al. Prediction of pathologic node-negative clinical stage IA lung adenocarcinoma for optimal candidates undergoing sublobar resection. J Thorac Cardiovasc Surg 2012;144:1365-71. [Crossref] [PubMed]
- Miyasaka Y, Suzuki K, Takamochi K, et al. The maximum standardized uptake value of fluorodeoxyglucose positron emission tomography of the primary tumour is a good predictor of pathological nodal involvement in clinical N0 non-small-cell lung cancer. Eur J Cardiothorac Surg 2013;44:83-7. [Crossref] [PubMed]
- Zhang Y, Sun Y, Xiang J, et al. A prediction model for N2 disease in T1 non–small cell lung cancer. J Thorac Cardiovasc Surg 2012;144:1360-4. [Crossref] [PubMed]
- Cho S, Song I, Yang HC, et al. Predictive factors for node metastasis in patients with clinical stage I non-small cell lung cancer. Ann Thorac Surg 2013;96:239-45. [Crossref] [PubMed]
- Shimamatsu S, Takenoyama M, Shimokawa M, et al. The influence of clinical T factor on predicting pathological N factor in resected lung cancer. Ann Thorac Surg 2019;108:1080-6. [Crossref] [PubMed]
- Sobin LH, Gospodarowicz MK, Wittekind Ch. TNM classification of malignant tumours. 7th edition. Chichester, West Sussex, UK; Hoboken, NJ: Wiley-Blackwell, 2010:138-46.
- Brierley J, Gospodarowicz MK, Wittekind Ch. TNM classification of malignant tumours. 8th edition. Chichester, West Sussex, UK; Hoboken, NJ: John Wiley & Sons, Inc., 2017:106-12.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453-7. [Crossref] [PubMed]
- Rusch VW, Asamura H, Watanabe H, et al. Members of IASLC Staging Committee, The IASLC Lung Cancer Staging Project: A proposal for a new International Lymph Node Map in the Forthcoming Seventh Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2009;4:568-77.
- Travis WD, Asamura H, Bankier AA, et al. International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee and Advisory Board Members, The IASLC lung cancer staging project: proposals for coding T categories for subsolid nodules and assessment of tumor size in part-solid tumors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2016;11:1204-23.
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [Crossref] [PubMed]
- Dhital K, Saundersb CAB, Seed PT, et al. [18F]Fluorodeoxyglucose positron emission tomography and its prognostic value in lung cancer. Eur J Cardiothorac Surg 2000;18:425-8. [Crossref] [PubMed]
- Al-Sarraf N, Gately K, Lucey J, et al. Clinical implication and prognostic significance of standardised uptake value of primary non-small cell lung cancer on positron emission tomography: analysis of 176 cases. Eur J Cardiothorac Surg 2008;34:892-7. [Crossref] [PubMed]
- Gupta NC, Tamim WJ, Graeber GG, et al. Mediastinal lymph node sampling following positron emission tomography with fluorodeoxyglucose imaging in lung cancer staging. Chest 2001;120:521-7. [Crossref] [PubMed]
- Cerfolio RJ, Ojha B, Bryant AS, et al. The role of FDG-PET scan in staging patients with nonsmall cell carcinoma. Ann Thorac Surg 2003;76:861-6. [Crossref] [PubMed]
- Li L, Ren S, Zhang Y, Guan Y, et al. Risk factors for predicting the occult nodal metastasis in T1-2N0M0 NSCLC patients staged by PET/CT: potential value in the clinic. Lung Cancer 2013;81:213-7. [Crossref] [PubMed]
- Okada M, Nishio W, Sakamoto T, et al. Prognostic significance of perioperative serum carcinoembryonic antigen in non-small cell lung cancer: analysis of 1,000 consecutive resections for clinical stage I disease. Ann Thorac Surg 2004;78:216-21. [Crossref] [PubMed]
- Ding N, Mao Y, Gao S, et al. Predictors of lymph node metastasis and possible selective lymph node dissection in clinical stage IA non-small cell lung cancer. J Thorac Dis 2018;10:4061-8. [Crossref] [PubMed]
- Haruki T, Aokage K, Miyoshi T, et al. Mediastinal nodal involvement in patients with clinical stage I non–small-cell lung cancer. J Thorac Oncol 2015;10:930-6. [Crossref] [PubMed]
- Asamura H, Nakayama H, Kondo H, et al. Lymph node involvement, recurrence, and prognosis in resected small, peripheral, non-small-cell lung carcinomas: Are these carcinomas candidates for video-assisted lobectomy? J Thorac Cardiovasc Surg 1996;111:1125-34. [Crossref] [PubMed]
- Casiraghi M, Travaini LL, Maisonneuve P, et al. Lymph node involvement in T1 non-small-cell lung cancer: could glucose uptake and maximal diameter be predictive criteria? Eur J Cardiothorac Surg 2011;39:e38-43. [Crossref] [PubMed]
- Watanabe S, Oda M, Go T, et al. Should mediastinal nodal dissection be routinely undertaken in patients with peripheral smallsized (2 cm or less) lung cancer? Retrospective analysis of 225 patients. Eur J Cardiothorac Surg 2001;20:1007-11. [Crossref] [PubMed]
- Veronesi G, Maisonneuve P, Pelosi G, et al. Screening-detected lung cancers: is systematic nodal dissection always essential? J Thorac Oncol 2011;6:525-30. [Crossref] [PubMed]
- Allen MS, Darling GE, Pechet TT, et al. ACOSOG Z0030 Study Group, Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 2006;81:1013-9; discussion 1019-20. [Crossref] [PubMed]
- Suzuki K, Koike T, Asakawa T, et al. Japan Lung Cancer Surgical Study Group. (JCOG LCSSG), A prospective radiological study of thin-section computed tomography to predictpathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol 2011;6:751-6. [Crossref] [PubMed]


