A nomogram to predict prognosis of patients with lung adenosquamous carcinoma: a population-based study
Introduction
Cancer is a major public health problem, and lung cancer has remained the leading cause of cancer death in recent years (1). Primary lung cancer has been classified into nine categories according to the 2004 World Health Organization (WHO) as small cell carcinoma, adenocarcinoma (ADC), squamous cell carcinoma (SCC), large cell carcinoma, sarcomatoid carcinoma, adenosquamous carcinoma (ASC), carcinoid tumor, salivary gland tumor, and miscellaneous tumors, in which pulmonary ASC is defined as a mixed type of tumor consisting of both glandular and squamous cell components with at least 10% a proportion of each type (2). ASC of lung cancer is an infrequent variant accounting for less than 4% of all non-small cell lung cancer (NSCLC) cases (3). Elder male smokers are reported to be a vulnerable population of ASC and the prognoses appear to be poorer than that of other types of NSCLC (4,5).
Current treatment options for ASC rely on the guidelines for NSCLC, where surgery represents the only effective means to cure ASC, and postoperative adjuvant chemotherapy is preferred for patients with stages IA–IIIB (4,6). Prognostic factors, such as certain characteristics of tumors, have effects on clinical outcomes of ASC patients according to previous retrospective reviews, which are too numerous and intensive to be integrated for the optimization of patient management (4-12). In this case, a statistical-based tool to quantify risk by considering factors of tumors, nomography, has been often used to predict the survival of certain types of cancer patients (13-15). Therefore, we used data derived from the Surveillance, Epidemiology, and End Results (SEER) database in this study to identify potential risk factors associated with survival of patients with ASC, to develop a nomogram to visually predict their survival.
Methods
Data extraction
Data with patients diagnosed as pulmonary ADC, SCC, or ASC between 2004 and 2016 were extracted from the SEER database (http://seer.cancer.gov/) database using SEER*Stat software, version 8.3.6 (https://seer.cancer.gov/seerstat/). Briefly, patients with ASC (ICD-O-3 8560/3: Adenosquamous carcinoma) were eligible for analyses. The inclusion criteria were as follows: (I) patients with complete survival data; (II) ASC patients were confirmed pathologically or immunohistochemically; and (III) patients with information of surgery, radiotherapy and chemotherapy. The exclusion criteria were as follows: (I) patients confirmed by autopsy; (II) patients with a follow-up time of 0 or unknown; (III) patients with unavailable TNM stage; and (VI) patients with unknown differentiation grade. We also collected data of ASC patients diagnosed and treated by us in our department between 2010 and 2014. Approval was waived by the local ethics committee because SEER is publicly available and de-identified, and we also obtained signed authorization and permission to access and use the dataset. Our study was also approved by The Institutional Review Committee of Zhongshan Hospital, Fudan University, Shanghai, China (approval number: B2019-232R). Informed consent forms were exempt.
The following information was extracted from the SEER database for each patient: patient demographics (age at diagnosis, race, and sex); characteristics of tumors [number in total, site/location, histological type, histological grade, tumor-node-metastasis (TNM) stage, and overall stage]; history of treatment (surgery, radiotherapy, and chemotherapy); and follow-up records (survival months and cause of death). Of note, tumor stages were reviewed manually according to the eighth edition of American Joint Committee on Cancer (AJCC) TNM staging system. Seventy percent of eligible patients derived from SEER database were randomly divided into a training cohort by R software version 3.4.3 (https://www.r-project.org/) and 30% of patients from the SEER as well as patients in our database were classified into two validation cohorts to externally validate the final nomogram.
Statistical analysis
Clinicopathological variables between pulmonary ASC, ADC, and SCC groups were analyzed using the Pearson’s chi-square test or the Wilcoxon rank sum test using SEER-derived data. Cumulative survival curves were constructed using the Kaplan-Meier method, and log-rank tests were used for the comparisons. Patient variables with prognostic values were identified using Cox proportional hazards regression with robust variance estimations and presented with odds ratios (ORs). Univariate analysis (UVA) and multivariate analysis (MVA) were utilized to identify potential significant prognostic factors for the entire training cohort, where a backward stepwise model with the Akaike information criterion was finally used. Besides, the ASC cancer-specific survival outcomes were used to perform competing risk model analyses. All statistical UVA and MVA were performed using SPSS statistical software for Windows, version 25.0 (IBM Corp; Armonk, NY, USA). The competing risk model analyses were performed using the R software.
A nomogram was constructed based on the results of UVA as well as MVA using R software and its packages, mainly including rms, Hmisc, and ggplot. Prediction error was estimated with 1,000 bootstrap samples and the model performance was internally evaluated by the concordance index (C-index) and calibration plots derived from regression analysis, indicating the accuracy to distinguish subject outcomes (16). The nomogram was further validated in the two validation cohorts with actual survival by comparing the nomogram-predicted probabilities. Statistical significance was set at a two-sided P value <0.05.
Results
Patient characteristics
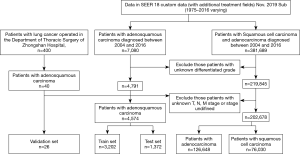
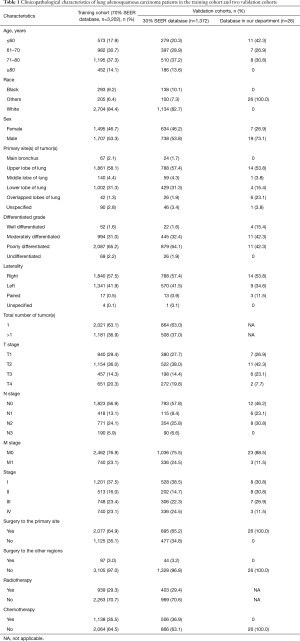
A total of 207,252 eligible patients with lung cancer were identified in the SEER database, incorporating 4,574 ASC, 126,648 ADC, and 76,030 SCC patients. A total of 26 ASC patients operated for primary lung cancer in the Department of Thoracic Surgery of the Fudan University (Zhongshan Hospital) were also included. Finally, 3,202 ASC patients from the SEER database overall were categorized into the training cohort. Two validation cohorts from SEER and our database consisted of 1,372 and 26 patients, respectively. The selecting process is shown in a flow diagram as presented in Figure 1. Characteristics of patients in the three cohorts are shown in Table 1.
Full table
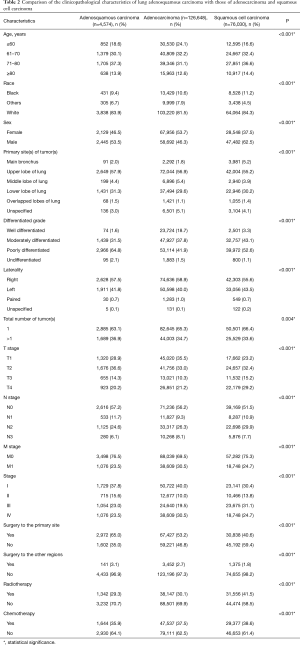
The number of eligible patients with ASC from the SEER database was only 3.6% of the number of patients with ADC and 6.0% of the number of patients with SCC. Male patients with ASC were slightly more in number than female patients (53.5% vs. 46.5%). The most common site of ASC was the upper lobe, with an occurrence of 57.9%. Significance was shown between the three histological subtypes in terms of patient age, race, sex, tumor site, total number of primary tumors, tumor histological grade, T stage, N stage, M stage, overall stage, surgery, radiotherapy, and chemotherapy (P<0.001).
The percentage of older patients with ASC were slightly higher than that of patients with ADC or SCC at their diagnoses. Tumors were presented with a lower grade of differentiation in ASC patients, 64.8% of which were poorly differentiated. Given results from the N stage and M stage, ASC tumors were less likely to show nodal and distant invasions than ADC and SCC tumors. Additionally, patients with ASC had a higher surgical rate compared to ADC and SCC patients, whereas the radiotherapy and chemotherapy percentages were relatively lower (Table 2).
Full table
Survival analysis
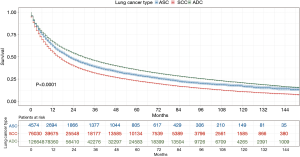
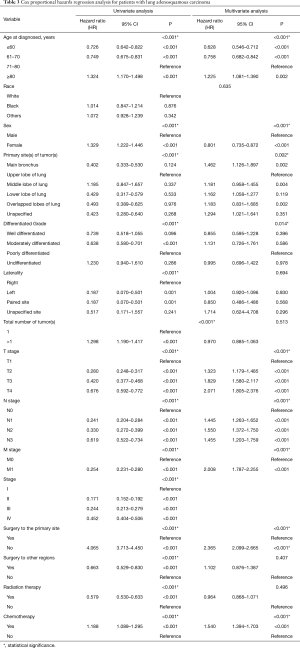
Patients with ASC were shown to survive significantly longer than those with SCC, but shorter than those with ADC (P<0.0001) (Figure 2). The median survival time of ASC, ADC, and SCC patients were 21.0 (19.3–22.7) months, 30.0 (29.6–30.4) months, and 16.0 (15.8–16.2) months, respectively. In the training cohort, patients were first included in an UVA to determine potential prognostic predictors for ASC. Finally, 14 variables, including patient demographics (age and sex), tumor characteristics (the site of primary tumor, laterality in lung, the number of tumors, histological grade, T stage, N stage, M stage, and overall stage), and patient history of treatment (surgery to primary site, surgery to other regions, radiotherapy, and chemotherapy), were shown to be significantly correlated with patient survival (P<0.001) (Table 3) (Figure S1A). No significant correlation was shown between patient race and their survival (P=0.635) (Figure S1B). Better survival outcomes were shown in patients who had undergone surgery or radiotherapy, whereas chemotherapy was significantly associated with a poorer prognosis.
Full table
Significant covariates in UVA (P<0.001) were furtherly analyzed in MVA. Overall stage was excluded in this process because it relied on the level of TNM stages that were included. The results revealed that nine of the variates were independent predictors for ASC patients, including age, sex, tumor site, histological grade, T stage, N stage, M stage, surgery to the primary site of tumors, and chemotherapy, while tumor laterality (P=0.694), number of tumors or sites in total (P=0.513), surgery to other regions (P=0.407), and radiotherapy (P=0.496) were not independent risk factors (Table 3). Contrary to the previous results, females were shown to have a significantly better prognosis than males with ASC (OR femalevs. male =0.801; 95% CI: 0.735, 0.872) using MVA, indicating that male sex was a negative factor for survival after excluding other mixed factors. Tumors in the main bronchus indicated poorer prognoses for patients than those with ASC in other sites. Given analyses among the four grades of tumor differentiation, better prognoses were shown in patients with moderately differentiated tumors than with poorly differentiated tumors. Results in terms of the TNM stage and chemotherapy were consistent with those obtained in the UVA. Furthermore, surgery to distant lymph nodes or sites or other regions was not as significantly beneficial as surgery to the primary site of tumor for ASC patients.
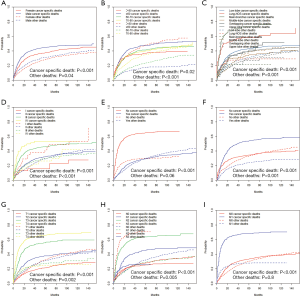
In order to make the comparisons of survival outcomes between groups more accurate, we also performed competing risk model analyses concerning the nine significant prognostic factors determined in the previous MVA with the cancer-specific survival outcomes in our training cohort. Results showed that patients with older ages, male genders, tumors located in the main bronchus, higher cell differentiation grades, higher T or N stages, or chemotherapy had significantly higher risks of both cancer-specific death from ASC (P<0.05) and other causes of death (P<0.05) (Figure S2). Patients in the groups with higher M stages or without surgery have significantly higher risks of cancer-specific death from ASC as well, but there was no significant difference in the probabilities of other causes of deaths (M stage: P=0.8; surgery: P=0.06) (Figure S2G,H).
Development and validations of the nomogram
A nomogram incorporating the nine independent risk factors (age, tumor histological grade, T stage, N stage, M stage, surgery, and chemotherapy) derived from MVA was developed (Figure 3A). Predicted 3-year and 5-year overall survival (OS) were calculated by identifying and summing up the point scales at the top of the nomogram of each factor. The 3-year and 5-year OS were obtained based on the point scale at the bottom of the nomogram. Internal evaluation was performed by bootstrap resampling and illustrated in calibration plots (Figure 3B,C). The C-index for prediction of 3-year and 5-year OS was 0.755±0.010, indicating the nomogram was in good agreement with the actual observation for ASC patients.
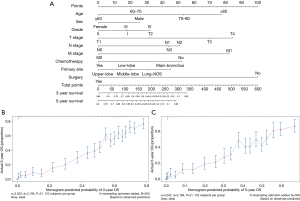
Furthermore, external evaluation of this nomogram was performed using the two validation cohorts derived from databases of SEER and ours. Given results from the comparison between nomogram-predicted survival and the actual survival of patients in the two validation cohorts, our nomogram showed reliability with a C-index of 0.748±0.049 (SEER database), and a C-index of 0.721±0.045 (database in our department), respectively. Calibration plots are presented in Figure 4.
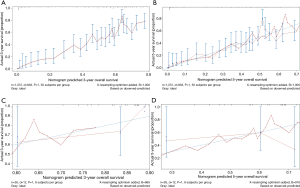
Besides, scores of each patient in the training cohort were calculated using our nomogram. The purpose of this step was to further validate the efficiency of our nomogram by comparing survival of patients grouped by the scores that obtained using the nomogram. Patients were first divided into two groups according to the median score. We estimated their survival by the Kaplan-Meier method, which was subsequently analyzed using a log-rank test. Results showed that there was a significant difference in the survival between these two groups of patients (P<0.001) (Figure 5A), where patients with predicted lower scores had indeed survived longer than those with higher scores. In addition, same methods were used among a four-group comparison of patients divided by the quartile of the score, and similar results were also obtained that their survival was significantly different (P<0.001) (Figure 5B).

In general, ASC patients who had a younger age, female sex, a relatively higher differentiated level, a lower T stage, a lower N stage, or a lower M stage had better clinical outcomes. Surgery to the primary site of tumors also led to superiority in patient survival, whereas chemotherapy was possibly to be pernicious. A nomogram was developed by integrating all the significant predictors above, so that survival of ASC patients was individually predicted according to their characteristics.
Discussion
In the current study we analyzed the risk factors for pulmonary ASC patients. Patient age, sex, tumor site, histological grade, T stage, N stage, M stage of the tumor, as well as surgery to the primary site of tumors and chemotherapy were independent prognosis factors based on analyses of more than 3,000 patients. A nomogram was finally developed to predict patient survival visually and reliably.
Characteristics of ASC patients, their tumors, and surgical percentages in our study were generally consistent with retrospective studies in earlier years. The average age of ASC patients at the time of diagnosis has been reported to be significantly higher compared to that of ADC patients (68.7 vs. 65.2 years; P<0.0001) and the ratio of males to females has been reported to be 3.38:1 among 114 ASC patients after surgery (4). Consistently, while patients with ASC and SCC had a similar age distribution, the median age of ASC patients was higher than that of ADC patients in the present study. The incidence of ASC was also higher in males than in females (male vs. female 53.5% vs. 46.5%). ASC has been reported to be more frequently peripheral than central and the size of central ASC tumors has been reported to be significantly larger than peripheral tumors (11). In the current study, consistent results were obtained showing that central ASC tumors only accounted for 2.0% of all the ASC tumors, but the comparison between central and peripheral tumors in terms of the size could not be determined. Surgery has been reported to be significantly performed more frequently in ASC patients than ADC patients (P=0.002) (4). In our study, approximately 65% of our patients were confirmed to have received surgery to primary tumor sites and 3.1% of the patients underwent surgical resections to other regions of tumors.
Patients with ASC have been reported to have significantly worse prognoses than those with either ADC or SCC, with their 5-year survival for all stages ranging from 6–33%, regardless of their treatments (4,5,8,17,18). However, survival results of ASC patients in our study showed an intermediate level between ADC and SCC patients, which differed from the previous reports. The differences might have resulted from bias, because patients in previous study comparisons were often all after surgery.
Similar to our study, several previous studies have reported ASC specific prognostic variables. Cell differentiation is one of the pivotal predictors for cancer, and a significant correlation has been observed between the differentiation of ASC cell types and patient survival (P<0.05) (8). Our study also showed that a lower grade of differentiation indicated poorer prognoses for patients, especially between moderate and poor grades of differentiation. The results of TNM stages of ASC in the present study were also consistent with tumor-stage associated variables reported in other studies. Tumor size (>5 cm), positive lymph node involvement, pleural invasion, and the presence of distant metastasis, as well as other tumor-stage associated variables that indicated higher stages of TNM have been reported to be significant ASC specific poor prognostic factors (9,10,12).
Surgery and chemotherapy are the two most studied treatment options for ASC. As reported, the postoperative 5-year survival rates of ASC patients for all stage cases were 23.3% and 54.6%, which were all shown to be significantly lower than those of patients with ADC or SCC of the lung (P<0.0001; P=0.017) (4,6). The 5-year survival rates of ASC patients for early-stage cases after surgery were reported to be 59.4%, and the survival of patients with stage I was significantly worse than that of ADC or SCC patients (P<0.0001), while there was no significance in postoperative survival among patients with these three pathological types for stage II cases (P=0.11) (19). According to the results from previous studies and ours, patients with lung ASC may benefit from surgery, but this is far less than that of patients with lung ADC or SCC for the same stage cases from surgery. We suppose that it is possibly attributed to the more aggressive nature of ASC compared with ADC and SCC of the lung. Besides, different effects have been reported for different surgical methods regarding the prognoses of ASC patients. Complete lobectomy was superior to segmental and partial resections for ASC patients in terms of their survival, but the difference was not significance (4,8), which was not validated in our study because the surgical procedure information in the SEER database for each patient was incomplete. Additionally, our study showed a decreased survival for patients who had undergone chemotherapy. However, both adjuvant chemotherapy (P<0.0001) and neoadjuvant chemotherapy (P<0.05) have been significantly advantageous for postoperative ASC patients (4,10). We therefore propose that chemotherapy should be combined with a surgical resection for ASC patients, to obtain better clinical outcomes.
Several studies have reported some other prognostic factors for ASC patients, which cannot be obtained and analyzed via data from the SEER. The pathological structure of ASC has been reported as a factor related to the survival of patients. ASC is divided into three subtypes by the proportions of the two components, ADC and SCC, as ADC-predominant ASC (the proportion of ADC ≥60% of tumor), SCC-predominant ASC (the proportion of SCC ≥60% of tumor) and structure-balanced ASC (the proportion of ADC and SCC is between 40% and 60%) (20). As indicated in previous studies, peripheral and central tumors are prone to be ADC- and SCC-dominant, respectively (21,22). Structure-balanced ASC have been found to have a significantly better prognosis for patients compared to its counterparts (P<0.05) (9,12). The genetic mutation status of various driver oncogenes has been examined in the ASC patient population to show that some of the genes are associated with prognosis. Activating mutations of the epidermal growth factor receptor (EGFR) have been reported in about 30–50% of ASC patients (23-28). Non-smokers (P=0.035) and lymphatic invasion positive patients (P=0.027) were significantly more prone to harbor this type of mutation (25). EGFR tyrosine kinase inhibitors (TKIs) have represented an effective treatment for ASC, with a reported objective response rate (ORR) of 26.5% and a disease control rate (DCR) of 65.3% (29). Patients with mutated EGFR tended to have an increased 3-year survival compared to those without the mutation, although the results were not significant (90.0% vs. 62.8%; P=0.06) (25). Kirsten rat sarcoma viral oncogene homolog (KRAS) mutations have also been reported in several studies with an incidence of approximately 5–10% (24,27,28,30), but almost no association has been reported between KRAS mutations and the prognoses of ASC patients.
To the best of our knowledge, our study is the largest populational-based retrospective study using data from the SEER for ASC patients. Our novel predictive model for prognoses of ASC patients was validated to be robustly reliable by both internal and external methods. However, there were still some limitations in our study. First, eligible patients derived from the SEER database were from the USA, which may not be relevant to other patient populations. Second, the lack of smoking history, as well as the absence of genetic mutations and other variables in the SEER records hindered the development of a more comprehensive prediction model for the survival of ASC patients. Third, a large randomized clinical trials (RCT) is a necessity to validate these results because our study was a retrospective design and confounding factors might have been introduced into the analyses of covariate effects. Novel and optimal treatment rationales will also be identified for ASC patients using this process.
Conclusions
Compared with ADC and SCC patients, ASC patients presented with distinct clinicopathological characteristics, including older age at diagnosis, lower grades of tumor differentiation, and lower incidences of nodal and distant invasions as well as higher percentages of surgical resections and lower percentages of chemotherapy or radiotherapy. Patient age, sex, tumor site, histological grade, T stage, N stage, M stage of the tumor, as well as surgery to the primary site of tumors and chemotherapy were shown to be independent prognostic factors based on the multivariate analyses. Using our nomogram, survival of each ASC patient could be predicted according to the clinicopathological characteristics.
Acknowledgments
The authors thank International Science Editing Co. (http://www.internationalscienceediting.com) for editing this manuscript.
Funding: This work was supported by the National Natural Science Foundation of China (Grant No. 81672268, 81572295; www.nsfc.gov.cn).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.115).The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethical statement: This work was approved by The Institutional Review Committee of Zhongshan Hospital, Fudan University, Shanghai, China (Approval Number: B2019-232R). Informed consent forms were exempt.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020. [Epub ahead of print]. [Crossref]
- World Health Organization classification of tumours, pathology and genetics. In: Tumours of the lung, pleura, thymus and heart. Lyon: IARC Press, 2004.
- Uramoto H, Yamada S, Hanagiri T. Clinicopathological characteristics of resected adenosquamous cell carcinoma of the lung: risk of coexistent double cancer. J Cardiothorac Surg 2010;5:92. [Crossref] [PubMed]
- Maeda H, Matsumura A, Kawabata T, et al. Adenosquamous carcinoma of the lung: surgical results as compared with squamous cell and adenocarcinoma cases. Eur J Cardiothorac Surg 2012;41:357-61. [Crossref] [PubMed]
- Nakagawa K, Yasumitu T, Fukuhara K, et al. Poor prognosis after lung resection for patients with adenosquamous carcinoma of the lung. Ann Thorac Surg 2003;75:1740-4. [Crossref] [PubMed]
- Mordant P, Grand B, Cazes A, et al. Adenosquamous carcinoma of the lung: surgical management, pathologic characteristics, and prognostic implications. Ann Thorac Surg 2013;95:1189-95. [Crossref] [PubMed]
- Hsia JY, Chen CY, Hsu CP, et al. Adenosquamous carcinoma of the lung. Surgical results compared with squamous cell and adenocarcinoma. Scand Cardiovasc J 1999;33:29-32. [Crossref] [PubMed]
- Riquet M, Perrotin C, Lang-Lazdunski L, et al. Do patients with adenosquamous carcinoma of the lung need a more aggressive approach? J Thorac Cardiovasc Surg 2001;122:618-9. [Crossref] [PubMed]
- Gawrychowski J, Brulinski K, Malinowski E, et al. Prognosis and survival after radical resection of primary adenosquamous lung carcinoma. Eur J Cardiothorac Surg 2005;27:686-92. [Crossref] [PubMed]
- Filosso PL, Ruffini E, Asioli S, et al. Adenosquamous lung carcinomas: a histologic subtype with poor prognosis. Lung Cancer 2011;74:25-9. [Crossref] [PubMed]
- Lee Y, Chung JH, Kim SE, et al. Adenosquamous carcinoma of the lung: CT, FDG PET, and clinicopathologic findings. Clin Nucl Med 2014;39:107-12. [Crossref] [PubMed]
- Zhao H, Yang H, Yao F, et al. Improved survival associated with a balanced structure between adenomatous and squamous components in patients with adenosquamous carcinoma of the lung. Eur J Surg Oncol 2016;42:1699-706. [Crossref] [PubMed]
- Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol 2015;33:861-9. [Crossref] [PubMed]
- Valentini V, van Stiphout RG, Lammering G, et al. Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J Clin Oncol 2011;29:3163-72. [Crossref] [PubMed]
- Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 2008;26:1364-70. [Crossref] [PubMed]
- Huitzil-Melendez FD, Capanu M, O'Reilly EM, et al. Advanced hepatocellular carcinoma: which staging systems best predict prognosis? J Clin Oncol 2010;28:2889-95. [Crossref] [PubMed]
- Naunheim KS, Taylor JR, Skosey C, et al. Adenosquamous lung carcinoma: clinical characteristics, treatment, and prognosis. Ann Thorac Surg 1987;44:462-6. [Crossref] [PubMed]
- Takamori S, Noguchi M, Morinaga S, et al. Clinicopathologic characteristics of adenosquamous carcinoma of the lung. Cancer 1991;67:649-54. [Crossref] [PubMed]
- Cooke DT, Nguyen DV, Yang Y, et al. Survival comparison of adenosquamous, squamous cell, and adenocarcinoma of the lung after lobectomy. Ann Thorac Surg 2010;90:943-8. [Crossref] [PubMed]
- Shimizu J, Oda M, Hayashi Y, et al. A clinicopathologic study of resected cases of adenosquamous carcinoma of the lung. Chest 1996;109:989-94. [Crossref] [PubMed]
- Ishida T, Kaneko S, Yokoyama H, et al. Adenosquamous carcinoma of the lung. Clinicopathologic and immunohistochemical features. Am J Clin Pathol 1992;97:678-85. [Crossref] [PubMed]
- Watanabe Y, Tsuta K, Kusumoto M, et al. Clinicopathologic features and computed tomographic findings of 52 surgically resected adenosquamous carcinomas of the lung. Ann Thorac Surg 2014;97:245-51. [Crossref] [PubMed]
- Kang SM, Kang HJ, Shin JH, et al. Identical epidermal growth factor receptor mutations in adenocarcinomatous and squamous cell carcinomatous components of adenosquamous carcinoma of the lung. Cancer 2007;109:581-7. [Crossref] [PubMed]
- Jia XL, Chen G. EGFR and KRAS mutations in Chinese patients with adenosquamous carcinoma of the lung. Lung Cancer 2011;74:396-400. [Crossref] [PubMed]
- Shiozawa T, Ishii G, Goto K, et al. Clinicopathological characteristics of EGFR mutated adenosquamous carcinoma of the lung. Pathol Int 2013;63:77-84. [Crossref] [PubMed]
- Powrozek T, Krawczyk P, Ramlau R, et al. EGFR gene mutations in patients with adenosquamous lung carcinoma. Asia Pac J Clin Oncol 2014;10:340-5. [Crossref] [PubMed]
- Wang R, Pan Y, Li C, et al. Analysis of major known driver mutations and prognosis in resected adenosquamous lung carcinomas. J Thorac Oncol 2014;9:760-8. [Crossref] [PubMed]
- Shi X, Wu H, Lu J, et al. Screening for major driver oncogene alterations in adenosquamous lung carcinoma using PCR coupled with next-generation and Sanger sequencing methods. Sci Rep 2016;6:22297. [Crossref] [PubMed]
- Song Z, Lin B, Shao L, et al. Therapeutic efficacy of gefitinib and erlotinib in patients with advanced lung adenosquamous carcinoma. J Chin Med Assoc 2013;76:481-5. [Crossref] [PubMed]
- Tochigi N, Dacic S, Nikiforova M, et al. Adenosquamous carcinoma of the lung: a microdissection study of KRAS and EGFR mutational and amplification status in a western patient population. Am J Clin Pathol 2011;135:783-9. [Crossref] [PubMed]