
Awake veno-arterial extracorporeal membrane oxygenation in patients with perioperative period acute heart failure in cardiac surgery
Introduction
Perioperative period refractory acute heart failure can occur in severe heart diseases, which include acute right ventricular failure, acute left ventricular failure, and biventricular failure (1). Refractory acute heart failure usually results in high mortality, and few patients recover without mechanical circulatory support (MCS) using only inotropic support (2,3). Extracorporeal membrane oxygenation (ECMO) has been applied as a backup MCS method for perioperative period heart failure to provide stable hemodynamic support (4). ECMO could be used as a temporary measure as a “bridge to recovery”, potentially improving patient outcomes due to cardiopulmonary support (5). In recent years, ECMO has become a standard-of-care procedure during the perioperative period for cardiac surgery (6). Perioperative heart failure patients can be maintained using an ECMO device for several days or weeks in the interim until recovery or heart transplantation (7,8). Although some centers have begun using ECMO on awake, non-intubated patients, venovenous ECMO (V-V ECMO) has recently been reported to be effective in patients suffering from respiratory failure, including those with acute respiratory distress syndrome, and is also helpful in treating lung transplantation patients. This method may offer several benefits over mechanical ventilation (9,10), but there is still insufficient data regarding veno-arterial ECMO (V-A ECMO) on perioperative heart failure patients in cardiac surgery (11). Apart from a few case reports, such as one on coronary angioplasty with awake ECMO (12), there is also a little research regarding the use of V-A ECMO on conscious perioperative heart failure patients in cardiac surgery. Our study, through evaluating heart failure perioperative patients in our center, found that awake V-A ECMO can be used successfully as a bridge to recovery or heart transplantation.
Methods
From December 2013 through November 2019, 40 patients underwent V-A ECMO in our hospital (Department of Cardiovascular Surgery, the First Affiliated Hospital of Harbin Medical University). The data from cases of coronary artery bypass grafting (CABG, n=18), heart transplantation (HTx, n=15), congenital heart disease (CHD, n=7) were collected and analyzed. Sedative drugs were appropriately applied when initiating and/or decannulating the ECMO device or according to requirements. Data, including demographics, medical history, baseline conditions, survival, complications, and ECMO parameters, were retrospectively collected and analyzed. To clearly evaluate the effect of awake ECMO, we divided the patients into 2 groups (the awake-ECMO group and the asleep-ECMO group) according to the ventilation and sedative used.
Ethics
The ethics committee approved the study of our hospital (20190988). Informed consent was not obtained from all patients, as this is a retrospective study.
Indicators for awake ECMO
(I) Patients had no pulmonary severe complications, including severe pneumonia, atelectasis, pleural effusion, bronchospasm, and pulmonary hemorrhaging before ECMO. (II) Patients had stable cardiopulmonary status under ECMO support, including being hemodynamically stable with moderate inotropic support, oxygenation was satisfactory (SVO2 >65% and SAO2 >95%), and lower mechanical ventilation parameter settings were permissive (PiP <20 cmH2O, PEEP <8 cmH2O, FiO2 <0.4). (III) Patients were conscious and could follow instructions. 4. The patients could be extubated during awake ECMO and satisfied the above conditions.
ECMO devices and settings
The ECMO devices included the Maquet Rotaflow RF 32 and the Quadrox PLS System (Maquet Cardiovascular, Fairfield, NJ, USA). The arterial cannulas (17–21 Fr) and venous cannulas (25–29 Fr) (Medtronic, USA) were chosen according to the patient’s body size. Surgery in the patients was performed peripherally using a cut-down technique (the right side of the femoral vein to the right femoral artery), and a distal limb perfusion catheter was established using a 6–7 Fr sheath (Medtronic, USA), thereby avoiding ischemic limb complications. The activated clotting time was maintained at 160–220 s, based on the Extracorporeal Life Support Organization (ELSO) anticoagulation recommendations. Cross-matching platelets were transfused when the platelet count was <10,000 cells/mm3 in order to avoid bleeding complications. Red blood cell suspensions were transfused with leucocyte filtration or irradiated blood when hematocrit <0.30. Adequate circulatory support was maintained using a pump, and the systemic perfusion index was monitored, including blood pressure, venous pressure, heart rate, lactic acid concentration, oxygenation, and blood gas analysis. Cardiac contractility and heart function were evaluated by echocardiogram. The dosage of positive inotropic drugs was decreased when specific clinical indicators were achieved. A heat exchanger was used to maintain moderate body temperature. The patients who were at high risk of developing acute kidney injury (such as oliguria or high blood creatinine) and fluid overload accepted ECMO plus continuous renal replacement therapy (CRRT). ECMO plus intra-aortic balloon pumping (IABP) was performed if left ventricular afterload increased during ECMO. Patients were slowly weaned off ECMO if the hemodynamic stability and the arterial blood gases were satisfactory. Swan-Ganz catheterization and transthoracic echocardiography were used to evaluate changes in cardiac function throughout the weaning process, while the dosage of inotropic agents was adjusted as required.
IMV devices and settings
ECMO and IMV were used in combination with sedative drugs as required. The haloperidol, midazolam, dexmedetomidine, or muscle relaxant would be used as needed to make agitated or anxious patients more comfortable. The ventilator was set according to the lung condition. If the patients had severe pulmonary complications, we would apply “lung rest strategies” to achieve lung recovery by small tidal volume (4–6 mL kg-1), low-frequency ventilation (8–10 breaths min-1), and low oxygen concentration (40–60%). Appropriate positive end-expiratory pressure (PEEP) (6–10 cmH2O) was maintained to prevent alveolar collapse. High peak airway pressure (>35 cmH2O) was avoided to prevent barotraumas.
Statistical analysis
Statistical analysis was performed using SPSS 11.0 software (SPSS Inc.; Tokyo, Japan). The continuous variables are presented as mean ± standard deviation or as median with interquartile range. Categorical variables are presented as frequency distributions (n) and percentages (%). All P values <0.5 were considered statistically significant.
Results
A total of 40 patients were included; 12 patients underwent awake ECMO without continuous IMV, 6 of whom maintained satisfactory consciousness and were able to receive oral intake during the ECMO run except for when undergoing the initiating and/or decannulating of ECMO; the others required short-term sedation and /or IMV to help them overcome early hemodynamic instability. Meanwhile, 28 patients underwent ECMO and IMV simultaneously due to hypoxemia, severe hemodynamic disorders, or neurological complications (such as agitation, delirium, mania, etc.). The total mean age was 50.4±12.88 years, with 10 females and 30 males. The total average duration of ECMO and IMV time was 157.77±140.02 vs. 171.14±131.91 hours, respectively. The mean lactate value decreased from 13.33±2.57 to 4.13±0.91 mmol/L 24 h after ECMO initiation in the awake ECMO group; conversely, it decreased from 12.93±2.66 to 6.87±2.91 mmol/L in the asleep group. The patient characteristics, complications outcome data are shown in Table 1. All patients received the right femoral vein to right femoral arterial ECMO support. Our data showed that awake ECMO patients had fewer complications and better outcomes compared to the ventilation patients (P<0.05). The weaning rate and survival rate were 100% vs. 18% and 75% vs. 11%, respectively; 3 patients died of septic shock after being weaned off ECMO in the awake group, while 25 patients died of refractory hemodynamic dysfunction, heart failure, incompletely corrected deformity, severe hypoxemia, septic shock, multiple organ failure, bleeding and blood coagulation disorders in the asleep group. Complications such as bleeding, pneumonia, hemolysis, and abdominal distension occurred less commonly in the asleep group compared to the awake group (P<0.05). The final discharge rate was 75% (9) in the asleep group vs. 11% (3) in the awake group. The follow-up result (>1 year) showed that 8 (67%) were still alive in the awake group, and 2 (8%) were still alive in the asleep group (Figure 1). The patients of both groups who died after discharge succumbed to cerebral hemorrhage.
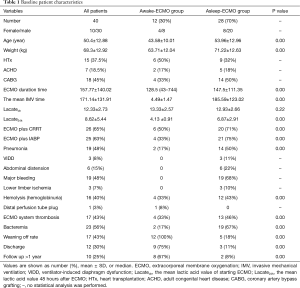
Full table
Discussion
V-A ECMO can provide 60–80% of predicted resting cardiac output, therefore, improve heart function during perioperative heart failure (13,14). ECMO support can be quickly established in acute heart failure patients in order to avoid further complications, such as pulmonary edema, hypoxemia, and multisystem organ failure, provided that the patients are good candidates for ECMO.
Choosing ECMO as opposed to a ventricular assist device (VAD)
Both ECMO and a VAD can be used for perioperative heart failure, but the ECMO is more suitable for our cases. First, heart failure tends to resolve within 2 weeks, and ECMO is an option for shorter durations. Secondly, in ECMO, it is easier to initiate and wean patients than in VAD, while it is the superior choice for emergency scenarios in the ICU. Thirdly, ECMO can provide oxygen and be converted to VAD at any time if necessary, which is beneficial for patients with lung injury or pneumonia. It is also worth mentioning that, due to economic constraints, most Chinese patients still choose ECMO as a short-term mechanical circulatory support device rather than VAD (15).
Benefits and disadvantages of awake ECMO
ECMO allows patients to breathe spontaneously and is superior to mechanical ventilation, thereby preventing the lung injury that is associated with sedation, intubation, and mechanical ventilation, as well as other complications, including overventilation, respiratory alkalosis, hypotension, barotraumas, upper gastrointestinal bleeding, respiratory-related pneumonia, and diaphragm dysfunction (16,17).
Of course, the opposition to this method is well known, with the detractors asserting that spontaneous ventilation-induced lung injury will occur during awake ECMO due to spontaneous breathing, generating positive transpulmonary pressure similar to that of mechanical ventilation. It is further claimed that strenuous respiratory muscle effort can lead to high oxygen consumption, which in turn could worsen hypoxemia (18). However, none of these phenomena were observed in our study. In our practice, we prefer to maintain spontaneous breathing due to the benefits it can provide. Awake ECMO can protect airways while avoiding the difficulty in handling airway secretions and hemodynamic complications. Allowing patients to move relatively freely and/or lie in a semi-recumbent position can reduce pulmonary complications, such as hypostatic pneumonia or atelectasis. Also, awake patients can follow rehabilitation exercise training instructions by doctors or caregivers, which could help to avoid muscle atrophy, deep vein thrombosis, and decubitus, while increasing lower limb venous and lymphatic return. Talking freely without intubation and sedation can help patients communicate, promoting mental health by reducing anxiety, irritability, or depression. Regular oral intake helps promote recovery in gastrointestinal function, which also decreases complications, such as abdominal bloating (19). However, this also presents challenges for ICU staff to manage conscious ECMO patients, especially in ensuring that all patient lines, catheters, and tubes are not disturbed while the patient can move their body. This strategy is not suitable for patients with mood disorders, as it can imperil patient safety. If no bleeding occurs, the activated clotting time value should be maintained at a moderate level to avoid oxygenator thrombosis (20).
Patient selection for V-A ECMO
There are a few studies available that report on awake V-A ECMO in perioperative acute heart failure, and most of these focus on refractory cardiogenic shock due to myocardial infarction, HTx, or pediatric cardiogenic shock (21,22). Our study emphasized those patients that had only perioperative period acute heart failure, including right heart failure or left heart failure in CABG, HTx, CAD (atrial and ventricular septal defect) patients. These patients usually have better outcomes via V-A ECMO assistance according to our experience and/or the literature. At the same time, these patients are more suitable for awake ECMO because the hemodynamics and system perfusion index values are improved after ECMO support. Totally and/or continuous awake ECMO is difficult to implement and rare due to the complexity of the conditions involved, and most awake cases still need short-term sedation and/or IMV as a bridge in order to overcome unstable hemodynamics in early acute heart failure patients.
Patients were excluded if they had severe/active bleeding, continually oozed blood, or had pericardium/pleural effusion due to reduced respiratory and circulatory stability. These conditions preclude awake and freely moving patients. ECMO patients must follow instructions by caregivers in order to keep all lines and tubes intact and connected. We do not recommend that awake ECMO be used to disturb critically ill patients, as catastrophic events may occur in patients who lack sufficient experience. In fact, in our study, almost all critically ill patients, including those with other severe organ complications, were in the asleep group. Some of these complications that occur in critical patients are difficult to resolve. Taken together, the reasons above could explain why we obtained better results in the awake ECMO group compared to the asleep group. Even so, we stress that it is worth encouraging awake ECMO if applied in patients complying with ECMO indications, as this can offer remarkable benefits. For instance, cannulation strategies may affect awake ECMO, and using a femoral-femoral method is not superior to single-site cannulation using a bicaval dual-lumen catheter. This kind of insertion at the internal jugular does not affect lower limb activity, and patients can walk with assistance (22). Our study lacked data related to the above because we did not have an available bicaval dual-lumen catheter. However, this is not a contraindication for mobilization via femoral catheters (23); instead, it can be performed with the requisite care and skill.
Our special focus on details of ECMO management
Awake ECMO management is crucial for successful weaning. The temperature is maintained between 36 and 36.5 °C. Patients can feel uncomfortable under hypothermic conditions, while high body temperatures increase oxygen consumption. Transfusion should be increased in order to raise oxygen levels (hematocrit >0.30) and maintain normal blood coagulation function, as there will be blood damage due to ECMO or bleeding. Red blood cell suspension with leukocyte filtration or irradiated blood and platelets by cross-matching test (platelet antibodies negative) is recommended for transfusion in order to avoid transfusion-associated graft-versus-host disease and refractoriness to platelet transfusion (24,25), especially in heart transplant patients and those who need many transfusions. Blood damage (based on erythrocytes, platelets, or clotting factors) may worsen due to ECMO along with continuous renal replacement therapy (CRRT) or intra-aortic balloon pump (IABP). Further studies have confirmed that the duration of ECMO is not associated with the development of thrombocytopenia, but decreased platelet dysfunction could induce coagulopathy and bleeding complications (26-28). In contrast, the extent of illness or platelet count can predict the development of severe thrombocytopenia while receiving ECMO. Pump flow should be altered to achieve adequate circulatory support and systemic perfusion. Some complications are inevitable, especially in a long-term ECMO duration. The thrombogenesis (including minor thrombosis of the oxygenator/connector/tube/centrifugal pump) improved as time went on, but most of oxygenator has not been changed, considering that the transmembrane pressure, oxygenation capacity, and blood coagulation function were not affected by thrombosis. Of course, the patient expense was another vital factor to consider. To reduce the occurrence of these adverse events, we constantly adjust the anticoagulation strategy in order to try to keep a balance between bleeding and thrombosis. Other essential details include the frequent flushing of the distal perfusion tube in order to avoid clogging.
Bacteremia and pneumonia occurred in a few patients. Most of symptoms and results improved markedly after antibiotics treatment according to bacteria culture and a drug-sensitive test of the blood of patients, and no patient needed further ventilation in the awake group. It is worth mentioning that CRRT should be connected to the ECMO circuit in order to reduce the puncture point and to allow the limbs to move freely. ECMO duration time is significant, as myocardial recovery generally requires 3–7 days. The more ECMO support time that is needed, the worse the resulting prognosis will be (29), although this conclusion requires further investigation. On the contrary, the Extracorporeal Life Support Organization (ELSO) data revealed that myocarditis and heart transplantation could achieve better outcomes despite longer support duration (30).
Special monitoring during awake ECMO
Vital signs and line security need to be closely monitored in awake ECMO patients (31), as patients can accidentally disturb or influence the security of the lines and cannulas. Accurate blood pressure and central venous pressure measurements are difficult to record because the patient can move, meaning that the volume status and respiratory function of each patient should be monitored carefully. Clinicians should be alert when abnormal breathing (such as respiratory rate, dyspnea rapid, shallow breathing, etc.) occurs during awake ECMO. Caregivers should attempt to ensure that patients are cooperative and comfortable during prolonged ECMO support in order to avoid adverse emotional events, including anxiety, irritability, or depression, which may occur due to long-term bed rest (32).
Limitations
This is a retrospective study, rather than a prospective, randomized, and comparative clinical study. Hence, selective bias might have occurred in the grouping, and we hope to conduct a prospective observational experiment to confirm the benefits of awake ECMO in the future.
Conclusions
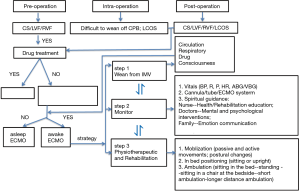
Awake V-A ECMO may be successfully applied in perioperative acute heart failure in cardiac surgery. The protocol and management details for awake ECMO are vital (Figure 1). The success criteria for awake ECMO include patient selection, procedure, ECMO duration, good teamwork for patient management, careful monitoring, and nursing.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.04.38). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The ethics committee approved the study of our hospital (20190988). Informed consent was not obtained from all patients, as this is a retrospective study. All personal data have been protected and secured according to current national and international laws.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Haneya A, Philipp A, Puehler T, et al. Temporary percutaneous right ventricular support using a centrifugal pump in patients with postoperative acute refractory right ventricular failure after left ventricular assist device implantation. Eur J Cardiothorac Surg 2012;41:219-23. [PubMed]
- Khorsandi M, Dougherty S, Bouamra O, et al. Extra-corporeal membrane oxygenation for refractory cardiogenic shock after adult cardiac surgery: a systematic review and meta-analysis. J Cardiothorac Surg 2017;12:55. [Crossref] [PubMed]
- Csepe TA, Kilic A. Advancements in mechanical circulatory support for patients in acute and chronic heart failure. J Thorac Dis 2017;9:4070-83. [Crossref] [PubMed]
- Stewart GC, Givertz MM. Mechanical circulatory support for advanced heart failure: patients and technology in evolution. Circulation 2012;125:1304-15. [Crossref] [PubMed]
- Rastan AJ, Dege A, Mohr M, et al. Early and late outcomes of 517 consecutive adult patients treated with extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. J Thorac Cardiovasc Surg 2010;139:302-11, 311.e1.
- Takeda K, Garan AR, Ando M, et al. Minimally invasive CentriMag ventricular assist device support integrated with extracorporeal membrane oxygenation in cardiogenic shock patients: a comparison with conventional CentriMag biventricular support configuration. Eur J Cardiothorac Surg 2017;52:1055-61. [Crossref] [PubMed]
- Gupta P, McDonald R, Chipman CW, et al. 20-year experience of prolonged extracorporeal membrane oxygenation in critically ill children with cardiac or pulmonary failure. Ann Thorac Surg 2012;93:1584-90. [Crossref] [PubMed]
- Tramm R, Ilic D, Davies AR, et al. Extracorporeal membrane oxygenation for critically ill adults. Cochrane Database Syst Rev 2015;1:CD010381. [PubMed]
- Kearns SK, Hernandez OO. "Awake" Extracorporeal Membrane Oxygenation as a Bridge to Lung Transplant. AACN Adv Crit Care 2016;27:293-300. [Crossref] [PubMed]
- Nosotti M, Rosso L, Tosi D, et al. Extracorporeal membrane oxygenation with spontaneous breathing as a bridge to lung transplantation. Interact Cardiovasc Thorac Surg 2013;16:55-9. [Crossref] [PubMed]
- Park BS, Lee WY, Lim JH, et al. Delayed Repair of Ventricular Septal Rupture Following Preoperative Awake Extracorporeal Membrane Oxygenation Support. Korean J Thorac Cardiovasc Surg 2017;50:211-4. [Crossref] [PubMed]
- Kass M, Moon M, Vo M, et al. Awake extracorporeal membrane oxygenation for very high-risk coronary angioplasty. Can J Cardiol 2015;31:227.e11-3. [Crossref] [PubMed]
- King CS, Roy A, Ryan L, et al. Cardiac Support: Emphasis on Venoarterial ECMO. Crit Care Clin 2017;33:777-94. [Crossref] [PubMed]
- Sun T, Guy A, Sidhu A, et al. Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) for emergency cardiac support. J Crit Care 2018;44:31-8. [Crossref] [PubMed]
- Rousse N, Juthier F, Pinçon C, et al. ECMO as a bridge to decision: Recovery, VAD, or heart transplantation. Int J Cardiol 2015;187:620-7. [Crossref] [PubMed]
- Borkowska M, Labeau S, Schepens T, et al. Nurses' Sedation Practices During Weaning of Adults From Mechanical Ventilation in an Intensive Care Unit. Am J Crit Care 2018;27:32-42. [Crossref] [PubMed]
- Seganfredo DH, Beltrão BA, Silva VMD, et al. Analysis of ineffective breathing pattern and impaired spontaneous ventilation of adults with oxygen therapy. Rev Lat Am Enfermagem 2017;25:e2954. [Crossref] [PubMed]
- Yoshida T, Uchiyama A, Matsuura N, et al. Spontaneous breathing during lung-protective ventilation in an experimental acute lung injury model: high transpulmonary pressure associated with strong spontaneous breathing effort may worsen lung injury. Crit Care Med 2012;40:1578-85. [Crossref] [PubMed]
- Langer T, Santini A, Bottino N, et al. "Awake" extracorporeal membrane oxygenation (ECMO): pathophysiology, technical considerations, and clinical pioneering. Crit Care 2016;20:150. [Crossref] [PubMed]
- Buckvold SM, Kinsella JP. Bleeding and Thrombosis in Pediatric Extracorporeal Membrane Oxygenation. Can We Improve Anticoagulation Strategies. Am J Respir Crit Care Med 2017;196:676-7. [Crossref] [PubMed]
- Liu S, Ravandi A, Kass M, et al. A Case Of Awake Percutaneous Extracorporeal Membrane Oxygenation For High-risk Percutaneous Coronary Intervention. Cureus 2017;9:e1191. [PubMed]
- Schmidt F, Jack T, Sasse M, et al. "Awake Veno-arterial Extracorporeal Membrane Oxygenation" in Pediatric Cardiogenic Shock: A Single-Center Experience. Pediatr Cardiol 2015;36:1647-56. [Crossref] [PubMed]
- Perme C, Nalty T, Winkelman C, et al. Safety and Efficacy of Mobility Interventions in Patients with Femoral Catheters in the ICU: A Prospective Observational Study. Cardiopulm Phys Ther J 2013;24:12-7. [Crossref] [PubMed]
- Chakrabarty R, Das SS. Successful surgical procedure in a patient of aplastic anemia with platelet refractoriness, using cross-match compatible platelets. Asian J Transfus Sci 2017;11:192-4. [Crossref] [PubMed]
- Wozniak MJ, Sullo N, Qureshi S, et al. Randomized trial of red cell washing for the prevention of transfusion-associated organ injury in cardiac surgery. Br J Anaesth 2017;118:689-98. [Crossref] [PubMed]
- Abrams D, Baldwin MR, Champion M, et al. Thrombocytopenia and extracorporeal membrane oxygenation in adults with acute respiratory failure: a cohort study. Intensive Care Med 2016;42:844-52. [Crossref] [PubMed]
- Saini A, Hartman ME, Gage BF, et al. Incidence of Platelet Dysfunction by Thromboelastography-Platelet Mapping in Children Supported with ECMO: A Pilot Retrospective Study. Front Pediatr 2016;3:116. [Crossref] [PubMed]
- Wu B, Gong D, Xu B, et al. Decreased platelet count in patients receiving continuous veno-venous hemofiltration: a single-center retrospective study. PLoS One 2014;9:e97286. [Crossref] [PubMed]
- Yeo HJ, Kim D, Jeon D, et al. Extracorporeal membrane oxygenation for life-threatening asthma refractory to mechanical ventilation: analysis of the Extracorporeal Life Support Organization registry. Crit Care 2017;21:297. [Crossref] [PubMed]
- Smith M, Vukomanovic A, Brodie D, et al. Duration of veno-arterial extracorporeal life support (VA ECMO) and outcome: an analysis of the Extracorporeal Life Support Organization (ELSO) registry. Crit Care 2017;21:45. [Crossref] [PubMed]
- Turner KM, Winder R, Campbell JL, et al. Patients' and nurses' views on providing psychological support within cardiac rehabilitation programmes: a qualitative study. BMJ Open 2017;7:e017510. [Crossref] [PubMed]
- Acevedo-Nuevo M, González-Gil MT, Romera-Ortega MÁ, et al. The early diagnosis and management of mixed delirium in a patient placed on ECMO and with difficult sedation: A case report. Intensive Crit Care Nurs 2018;44:110-4. [Crossref] [PubMed]


