
Clinical long-term outcome of non-specific pleuritis (NSP) after surgical or medical thoracoscopy
Introduction
Pleural effusion is a manifestation of a variety of diseases benign or malignant. After thorough clinical work-up that extends from clinical history to diagnostic thoracentesis and imaging techniques, many patients will finally undergo thoracoscopy either for diagnostic purposes, in this case mostly searching for malignancy, and/or for therapeutic ones, in order to proceed to pleural adhesions lysis in inflammatory conditions or to create them by pleurodesis in neoplastic cases (1,2). Thoracoscopy can be performed by the respiratory physician [medical thoracoscopy (MT), or pleuroscopy] under local anesthesia and spontaneous ventilation, or by the surgeon (video-assisted thoracoscopic surgery-VATS) under general anesthesia and selective ventilation, and no randomized trials have compared the two techniques (3).
When performing pleural biopsies during thoracoscopy, the diagnosis of malignancy will guide treatment decisions and patient’s prognosis. However, in a substantial proportion of cases, almost 20% of thoracoscopies (4), a descriptive histologic diagnosis of inflammation and/or fibrosis will be made, clinically encompassing the non-specific pleuritis (NSP) (5). NSP is not easy to define, as it is the histological presentation of most of the over 50 causes of benign pleural disease, except for malignancy and tuberculosis (4). However, and despite the non-specific histological diagnosis, clinicians that will thoroughly go through all data will arrive at a precise etiological diagnosis in most of the cases, leaving some cases to be considered as “idiopathic” (6), and similarly careful examination of NSP histology could reveal some patterns of underlying disease (7).
Nevertheless, the major question after NSP diagnosis will be a potential overlooked malignancy. Previous studies reporting on the possibility of an overlooked malignancy after NSP diagnosis on pleural biopsy have shown up to 15% false-negative diagnoses (8). Yet, in case of malignant pleural effusion, both surgical and medical techniques have a high diagnostic yield up to 92% (8). However, a comparison between “medical” or “surgical” thoracoscopy on this field has not been performed.
Thus, the aim of this study is to report the long-term outcome of patients initially diagnosed with NSP, after medical or surgical techniques, in order to report possible diagnostic errors (false-negative cases) in this patient population.
Methods
Study group
This is a retrospective study of consecutive patients with recurrent pleural effusion subjected to parietal pleura biopsy from January 2011 to January 2017. It includes two groups: (I) a group of patients (n=179) diagnosed with NSP by video-assisted thoracoscopic surgery (VATS) at the University Hospital of Saint-Etienne, France and (II) a group of patients (n=116) after MT at the University Hospital of Alexandroupolis, Greece. The study was approved by the Internal Review Board of the University Hospital of Saint-Etienne, France (212018/CHUSTE) and the Internal Review Board of the University Hospital of Alexandroupolis, Greece (IRB2/19-2-2014).
Inclusion criteria were a histological diagnosis of NSP excluding cases of neoplastic or granulomatous disease and a minimum follow up of 12 months, as suggested for these cases to ascertain the benign etiology (9). Medical files were reviewed to record demographic data, clinical features, clinical history and all available laboratory and investigational data during the diagnostic work-up of pleural effusion.
Statistical analyses were performed using StatViewTM 4.5 software (Abacus Concepts Inc., Berkeley, Calif., USA). Values are expressed as mean ± SD or number and percentages. Comparison of means between groups was performed with Student’s t-test. The χ2 test was used to determine whether a difference existed between the demographic parameters. Negative predictive value (NPV) is the probability that the disease is not present when the test is negative = d/(b + d) where b is the false negative and d the true negative.
Results
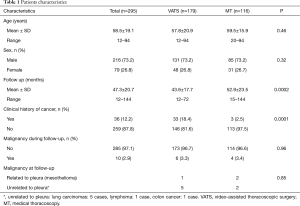
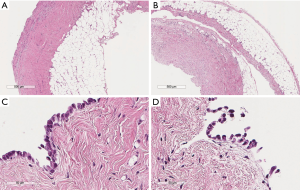
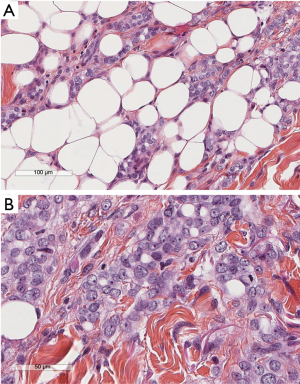
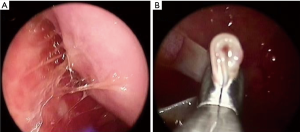
Patients’ characteristics are shown in Table 1. Sex and age data were similar between the two groups. Mean overall follow up period (Table 1) was 47.3±20.7 months and it was significantly longer (P=0.002) for the MT than for the VATS group (52.9±23.5 vs. 43.6±17.7 month, respectively). Patients with clinical history of cancer were overall 36 (12.2%), significantly more (P=0.0001) in the VATS group than the MT group. Overall, 10 patients (2.95%) were diagnosed with a malignancy after thoracoscopy: 6 patients from the VATS group, and 4 from the MT group. Three patients (1.01%), one from the VATS group and two from the MT group, did develop pleural malignancy. One female patient (VATS group) with a pleural biopsy of 5.5 cm added maximal diameter (AMD) had a diagnosis of atypical mesothelial hyperplasia (AMH) (Figure 1) at the age of 58; this diagnosis was confirmed by an external malignant pleural mesothelioma (MPM) panel (MESOPATH). She developed MPM 5 years later (Figure 2). In the MT group (Figure 3), two men of 70 and 71 years old, developed MPM at 62 and 64 months after the initial biopsy. The initial specimens, measuring up to 2 and 3 cm of AMD have shown chronic inflammation, fibrosis, and mild mesothelial cell hyperplasia.
Full table
In the VATS group (Table 1), the diagnosis of lung cancer was established in two patients one month later by bronchial biopsies: one patient had a “suspicious” pleural fluid cytology, but biopsies showed no malignancy. The pleural specimen was adequate measuring up to 8 cm AMD and deep enough including adipose tissue. The second with AMD 3.5 cm underwent pneumonectomy and large pleural biopsies taken were also negative for malignancy. A third patient, who had a known small B-cell lymphoma, his pleural biopsies (7 cm AMD) showed no recurrence. In this patient recurrence was found 18 months later, in the newly developed mediastinal lymph nodes. A fourth patient had a known gastric cancer and developed colon carcinoma 3 years later. Pleural biopsy (AMD 5 cm) was negative. A fifth patient with negative pleural biopsy developed lung cancer 5 years later. Respectively from the MT group, 2 patients were diagnosed with centrally located lung carcinoma by bronchoscopy within the first month after the procedure (Table 1).
Considering only the related to pleura malignancies, NPV for VATS =0.994 and for MT =0.982 and if we consider all malignant cases, NPV still remains high for VATS =0.966 and for MT =0.965.
Discussion
In the current series, which is one of the largest series reporting in the outcome of NSP, thoracoscopy, surgical and medical, proved to be an excellent diagnostic technique with high diagnostic accuracy in excluding malignancy as VATS had a NPV up to 0.994 and MT up to 0.982 translating a high specificity yield. There are no studies directly comparing medical with surgical thoracoscopy (3,10), in any of diagnostic, therapeutic, outcome or cost-effectiveness fields. NSP encompasses many different patterns that could suggest a possible underlying etiology (7). Our group has recently shown that a severely fibrotic pleura with cellular fibrosis and neutrophils is more representative of bacterial disease, lymphocyte-predominant inflammation with mild collagenous fibrosis is mostly associated with viral disease, moderate collagenous fibrosis with variable degree of lymphocytic inflammation is seen in heart disease, important cellular fibrosis with moderate lymphocytic inflammation is found in autoimmune disease, while recurrent pneumothorax is characterized by numerous histiocytes and eosinophils, and by no or mild fibrosis (7).
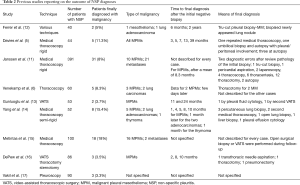
However, when the “non-specific” diagnosis of pleuritis is made from the pathologist, then the real and most worrisome question is whether a truly benign condition involves the pleural cavity, or this is a false-negative diagnosis. This latter scenario of false-negative diagnosis could have a few explanations; first, a sampling error which can be attributed to a thickened fibrous pleura complicating the biopsy procedure or multiple adhesions not allowing the full cavity inspection and thus the proper site selection to perform the biopsy (11). In the study of Janssen et al. (11), 31 out of 208 cases finally revealed malignancy (Table 2), diagnosed after a mean follow up of 4.4 months. Most of false-negative cases were considered as sampling errors, complicated by the aspect of the pleural cavity not allowing for proper inspection, like in the presence of adhesions or a fibrinous layer over the pleura (11). Not surprisingly, in this study the majority of the cases were MPM (11). The same was true in the study of Metintas and collaborators (15), where from the 18 inconclusive cases, 16 presented finally MPM. Venekamp et al. (6) referred their patients for thoracotomy few days after the first non-diagnostic biopsy, to finally diagnose MPM in two cases. It is well known that in order to make the diagnosis of MPM, one of the major histologic criteria is to prove the invasion into the underlying adipose tissue (18,19), but if the pleura is really thickened, then it might be difficult to obtain deeper biopsies. To avoid this problem, “biopsy into biopsy” is proposed by physicians, meaning performing deeper and deeper biopsies at the same spot (4). In case of “difficult thoracoscopy” and suspicion of MPM (asbestos exposure) and/or pleural plaques, thoracotomy may indeed give the answer (6).
A histopathologic diagnostic error due to the inherent difficulty of pleural diagnostics (18,19), might be another explanation. In order to perform the correct diagnosis, a proliferative lesion with underlying adipose tissue invasion should be found. However, some tumors like MPM and especially of the desmoplastic type, will not have excessive cellularity and frank invasion will not be always identifiable. This is true even for metastatic tumors which sometimes can be rather paucicellular, in this case posing troubles mostly during intra-operative frozen sections (20). On the other hand, there may be a rather florid mesothelial hyperplasia or a fibrosing pleuritis looking suspicious for malignancy but being reactive. In order to overcome this problem, p16 FISH and BAP1 immunohistochemistry have been used in the recent years to achieve a more confident diagnosis regarding MPM. More specifically, p16 homozygous deletion and loss of BAP1 expression are 100% specific for malignant MPM, albeit of relatively limited sensitivity (21).
Another explanation of a false-negative diagnosis is that the biopsy was not actually false-negative at the time of the initial diagnosis, but it rather represented the natural history of the disease. In our series, among the 295 patients with an NSP diagnosis, three (1.01%) were diagnosed with pleural malignancy over five years after the initial investigation, performing a second thoracoscopy. Again and despite the fact that in these patients pleural effusion was fully regressed during the follow-up period, these cases are the only that may raise questions about the accuracy of the investigation, although the natural history of MPM is unknown, and a possible long spontaneous evolution cannot be excluded. It is well known that chronic inflammation in the pleura may generate malignancy (19).
In the studies examining the outcome of NSP (Table 2) there are two patients, one from the series of Gunluoglu et al. (13) and one from the series of Davies et al. (5), that showed late MPM diagnosis involving the pleura, at 24 and 39 months, respectively. For these patients who will develop MPM several years later, the pathogenesis and natural history of MPM must be considered. Unlike most cancer types, a precursor lesion has not yet been defined for MPM. Could some cases of AMH represent an early form of this neoplasia? AMH is a term used when the definite nature, neoplastic or not, cannot be ascertain. Data from the Group Mesopath on 67 patients diagnosed with AMH showed a 60% 3-year survival signifying the inability of predicting the outcome of these cases (22). Interestingly, the basis of pre-invasive mesothelial neoplasia begins to be elucidated with the aid of ancillary techniques. Thus, specialized in MM pathologists from various centers collected ten cases of MM suggested to be in situ based on the following criteria: a surface single-layer of mesothelial cells with BAP1 loss, no invasive tumor by imaging and/or direct visual inspection of the pleura/peritoneum and no invasive MM diagnosed for at least 1 year after the biopsy, so as to avoid an actually suboptimal sample/false-negative diagnosis (23,24). Among these ten patients, seven developed invasive malignant MM with time between initial biopsy and invasive disease from 12 to 92 (median 60) months; invasive MM had not developed in the other three patients at 12, 57, and 120 months of follow-up (23,24). Furthermore, patients with MPM of the epithelioid subtype have a median survival of 16 months, but this further depends on detailed histological features, such as the mitotic index, the degree of nuclear atypia, and the presence of necrosis (25). When these features are low-grade, a survival of up to two or three years can be seen. Thus, initial NSP diagnoses in patients later developing malignant MPM could potentially represent false-negative diagnoses due to sampling/diagnostic error of low-grade tumors showing slower progression or early forms of mesothelial neoplasia.
In our series, the three MPM cases (2 for MT and 1 for VATS) were diagnosed over five years after the initial investigation by the same techniques, whereas among the other 7 cases finally revealing a malignancy, none showed initially pleural involvement of the disease. More specifically, in the VATS group the 3 patients (the lymphoma 18 months later, the colon carcinoma 3 years later and the lung carcinoma 5 years later) were unlike to present a pleural disease related to their malignancy. The remaining 4 patients diagnosed shortly after the pleural biopsy with lung cancer in non-pleural material (2 for VATS and 2 for MT), were unlike to have pleural malignancy, as in one of these, pneumonectomy with large parietal pleura biopsies did confirm the absence of pleural metastasis and the remaining 3 also had large pleural biopsies, suggesting a paramalignant effusion as they presented with central obstruction and the final diagnosis was made by bronchoscopy (1). Ferrer et al. (12) (Table 2) in one of their two cases, the diagnosis of lung carcinoma by biopsy of a lung nodule was made 2 years after the initial investigation and thus true pleural malignancy at the initial biopsy was not retained in this case. Because of a benign course in 85% of NSP patients actually is recommended (9) at least 12 months follow-up to confirm, yet according to the recent reports a thorough clinical history associated to the histological findings may diagnose all benign cases (7) and therefore “idiopathic pleural effusion” should not be retained as final diagnosis (6,26). Repeated or further interventions are indicated in case of persistent chest pain and/or recurrence of pleural effusion, or when CT scan findings are suggestive of malignant pleural disease (4).
Full table
Our study has certain limitations. This is a retrospective study with data collected retrospectively from single centers. Given this, we were dependent on clinical information in the medical records, with the unavoidable limitations within this context. Although the long follow-up of our patients and the absence of recurrence of pleural effusion suggest a benign cause, the specific diagnosis of some cases may be missing and there was no clue to recover data either from history or histological findings for specific diagnosis as suggested (7). Our patient population in both groups had the same distribution in age and sex, yet differences were observed in the follow-up period and clinical history of cancer between the two groups. These differences might be random or related indeed to the retrospective character of our study as well as to local policy between the two institutions in following this patient population and selecting patients for thoracoscopy.
To conclude, our study shows the similar and excellent negative predicted value of both MT and VATS in excluding malignant pleural disease and highlights the rare phenomenon of developing MPM many years after an initial non-malignant diagnosis, representing maybe the earlier forms of mesothelial neoplasia.
Acknowledgments
The authors would like to thank Mr Philippe Cosmo from the Tumorothèque/Centre de Ressources Biologiques de CHU Saint-Etienne (BRIF no. BB-0033-00041) for his assistance.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-19-3496). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Internal Review Board of the University Hospital of Saint-Etienne, France (212018/CHUSTE) and the Internal Review Board of the University Hospital of Alexandroupolis, Greece (IRB2/19-2-2014).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Froudarakis ME. Diagnostic Work-Up of Pleural Effusions. Respiration 2008;75:4-13. [Crossref] [PubMed]
- Anevlavis S, Froudarakis ME. Advances in pleuroscopy. Clin Respir J 2018;12:839-47. [Crossref] [PubMed]
- Bibby AC, Maskell NA. Pleural biopsies in undiagnosed pleural effusions; Abrams vs image-guided vs thoracoscopic biopsies. Curr Opin Pulm Med 2016;22:392-8. [Crossref] [PubMed]
- Janssen J, Maldonado F, Metintas M. What is the significance of non-specific pleuritis? A trick question. Clin Respir J 2018;12:2407-10. [Crossref] [PubMed]
- Davies HE, Nicholson JE, Rahman NM, et al. Outcome of patients with nonspecific pleuritis/fibrosis on thoracoscopic pleural biopsies. Eur J Cardiothorac Surg 2010;38:472-7. [Crossref] [PubMed]
- Venekamp LN, Velkeniers B, Noppen M. Does “idiopathic pleuritis” exist? Natural history of non-specific pleuritis diagnosed after thoracoscopy. Respiration 2005;72:74-8. [Crossref] [PubMed]
- Karpathiou G, Hathroubi S, Patoir A, et al. Non-specific pleuritis: pathological patterns in benign pleuritis. Pathology 2019;51:405-11. [Crossref] [PubMed]
- Bibby AC, Dorn P, Psallidas I, et al. ERS/EACTS statement on the management of malignant pleural effusions. Eur Respir J 2018;52:1800349. [Crossref] [PubMed]
- Bhatnagar R, Corcoran JP, Maldonado F, et al. Advanced medical interventions in pleural disease. Eur Respir Rev 2016;25:199-213. [Crossref] [PubMed]
- Shojaee S, Lee HJ. Thoracoscopy: medical versus surgical-in the management of pleural diseases. J Thorac Dis 2015;7:S339-51. [PubMed]
- Janssen J, Ramlal S, Mravunac M. The Long-Term Follow Up of Exudative Pleural Effusion After Nondiagnostic Thoracoscopy. J Bronchol 2004;11:169-74. [Crossref]
- Ferrer JS, Munoz XG, Orriols RM, et al. Evolution of idiopathic pleural effusion: a prospective, long-term follow-up study. Chest 1996;109:1508-13. [Crossref] [PubMed]
- Gunluoglu G, Olcmen A, Gunluoglu MZ, et al. Long-term Outcome of Patients With Undiagnosed Pleural Effusion. Arch Bronconeumol 2015;51:632-6. [PubMed]
- Yang Y, Wu YB, Wang Z, et al. Long-term outcome of patients with nonspecific pleurisy at medical thoracoscopy. Respir Med 2017;124:1-5. [Crossref] [PubMed]
- Metintas M, Ak G, Cadirci O, et al. Outcome of patients diagnosed with fibrinous pleuritis after medical thoracoscopy. Respir Med 2012;106:1177-83. [Crossref] [PubMed]
- DePew ZS, Verma A, Wigle D, et al. Nonspecific Pleuritis: Optimal Duration of Follow-Up. Ann Thorac Surg 2014;97:1867-71. [Crossref] [PubMed]
- Vakil E, Ost D, Vial MR, et al. Non-specific pleuritis in patients with active malignancy. Respirology 2018;23:213-9. [Crossref] [PubMed]
- Karpathiou G, Peoc’h M. Pleura revisited: From histology and pathophysiology to pathology and molecular biology. Clin Respir J 2019;13:3-13. [Crossref] [PubMed]
- Karpathiou G, Stefanou D, Froudarakis ME. Pleural neoplastic pathology. Respir Med 2015;109:931-43. [Crossref] [PubMed]
- Karpathiou G, Froudarakis M, Forest F, et al. Frozen sections in pleural pathology: a valuable tool. Respiration 2017;94:45-51. [Crossref] [PubMed]
- Churg A, Sheffield BS, Galateau-Salle F. New Markers for Separating Benign From Malignant Mesothelial Proliferations: Are We There Yet? Arch Pathol Lab Med 2016;140:318-21. [Crossref] [PubMed]
- Churg A, Galateau-Salle F. The Separation of Benign and Malignant Mesothelial Proliferations. Arch Pathol Lab Med 2012;136:1217-26. [Crossref] [PubMed]
- Churg A, Galateau-Salle F, Roden AC, et al. Malignant mesothelioma in situ: morphologic features and clinical outcome. Mod Pathol 2020;33:297-302. [Crossref] [PubMed]
- Churg A, Hwang H, Tan L, et al. Malignant mesothelioma in situ. Histopathology 2018;72:1033-8. [Crossref] [PubMed]
- Habougit C, Trombert-Paviot B, Karpathiou G, et al. Histopathologic features predict survival in diffuse pleural malignant mesothelioma on pleural biopsies. Virchows Arch 2017;470:639-46. [Crossref] [PubMed]
- Archontogeorgis K, Anevlavis S, Zarogoulidis P, et al. Pleuroscopy in “Idiopathic” eosinophilic pleural effusions. Clin Respir J 2015;9:475-80. [Crossref] [PubMed]


