
The effect of endoscopic vein harvesting in coronary artery bypass surgery
Introduction
Coronary artery bypass grafting (CABG) is considered as a better revascularization modality in most of the cases with multi-vessel coronary artery disease (1). Arterial grafts have been advocated because of their long-term patency compared with saphenous vein grafts (SVG) (2). However, arterial grafts are limited due to the occurrence of spasms, and poor results when the target vessels were less stenotic. SV remains an indispensable conduit in CABG. Endoscopic harvesting of SV is one of the novel innovative strategies in CABG during the last twenty years. But the impact of endoscopic vein harvesting (EVH) on CABG outcomes remains ambiguous (3). At the same time, it is challenging to treat the occluded SVG. Therefore, preventing SVG occlusion is of great importance (4). Hence, we aimed to compare clinical outcomes 1-year graft patency rates, the endothelial integrity, and postoperative wound complications between SV grafts accessed by EVH and open vein harvesting (OVH).
Methods
Study population
A total of one hundred coronary artery disease subjects who signed the informed consent form and permitted their SV to be preserved for studying from July 2015 to December 2015 were reviewed. SVG were harvested with EVH technique (EVH group) in 50 of them, while others accepted OVH technique (OVH group). All the patients underwent elective CABG with median sternotomy approach. And all of them had no less than 1 bypass graft aimed to diagonal branch or left circumflex artery planned to use SVG for conduit. This study was approved by the institutional review board and was performed in accordance with the Declaration of Helsinki and approved guidelines (Approval No.: 2015-652).
Vein harvest technique
We adopted the device of Maquet (Maquet Cardiovascular LLC, San Jose, CA, USA). A 2 cm skin incision was made directly across the SV, posteriorly and inferiorly to the medial tibial condyle. The balloon port was inserted to seal the incision to create a tunnel in the thigh. Then the fascia roofed over the vein was dissected to develop a space superior to the great SV. After that, the lateral and the inferior fascia were dissected. In order to protect the adventitia, the SV should be handled with care and avoid the dissector pointing towards the wall of the SV. The branches should be divided with a distance of more than 3 mm from the trunk. The SV was then detached near the saphenofemoral junction through a 3 mm incision. Following the removal, the distal end of the vein was cannulated. Then saline containing papaverine and heparin was injected into the vein to check the leaks. The branches were clipped with titanium clips. Repairing of the leakages was performed using 7-0 Prolene sutures. After evacuating the blood in the tunnel of the leg, the incision was closed with a continuous suture. The operated leg was then wrapped with an elastic bandage for 24 hours (Video 1).
OVH was conducted with a continuous incision along the route of the vein. An incision was made at the front of the malleolus. Care must be taken to avoid any injury of the saphenous nerve, vein and its branches. The wound was closed with continuous sutures. After vein harvesting, bandage was applied to the leg for 2 days.
Specimens and preparation for scanning electron microscope (SEM)
A 5-mm segment was cut-off from the end of the SV before distension and then fixed by 2% paraformaldehyde. After distention with heparinized papaverine saline, another 5-mm segment was taken from the end of the SV for fixing. Those specimens were post-fixed in 1% osmium tetroxide in phosphate buffer. Then the specimens were spatter-coated with a gold layer and observed with a JEOL JSM5510 SEM.
Follow-up
Follow-up data was obtained through clinic visits and telephone questionnaires at 3 months and 1 year postoperation. Clinical or telephone follow-up was closed on February 28, 2017. The primary outcome was the major adverse cardiac events (MACE). MACE included cardiac death, acute myocardial infarction, repeated coronary revascularization (5). Operative death was defined as death within 30 days postoperation.
Wound assessment
Wound healing was assessed from the day 1 to 6 weeks postoperation. Serious wound complications were defined as numerous serous exudates, purulence, isolation of bacteria, disruption of wound, additional treatment required and prolonged hospital stay. Numerous serous exudates were measured as more than 10% of the length of the wound involved. Additional treatment included upgradation of antibiotics due to leg wound, drainage of pus under local anesthesia and debridement of wound under general anesthesia (6).
Pain assessment
Leg pain was assessed using a Visual Analog Scale (7). The scale was a 100 mm evenly divided plain line with two end points of “no pain at all” and “worst imaginable pain”. The degree of pain was determined as the length of the line. Patients completed the pain score on the day discharge or 7 days postoperation (8).
Evaluation of graft patency
The coronary multidetector computed tomography angiogram (CCTA) was performed 1-year after operation regardless of angina based on the patient’s consent or when the postoperation patient wanted. Some patients underwent this examination before 1-year postoperation because of recurrence of angina. One physician reviewed all the CCTA scans no matter the examination conducted in our Hospital or other hospital. Because the left internal mammary artery to the left anterior descending branch is adopted in all patients, and grafts to the right coronary artery have a worse patency rate. The SV grafts to the diagonal branch and left circumflex coronary artery (LCx), including all the obtuse marginal and ramus branches, were studied. Patency was defined as less than 70% stenosis and occlusion was defined as greater than 70% stenosis (9).
Statistical analysis
Continuous data were expressed as means ± standard deviation and compared using Student t-test or analysis of variance. If the continuous data were not normally distributed, quartile and Mann-Whitney U test were selected. Categorical data were expressed as percentages and compared with chi-square test. Fisher’s exact test was adopted when low rates existed. All P values were two sided, and P<0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 20 (IBM SPSS Inc., Chicago, IL, USA).
Results
Table 1 presented the baseline characteristics of the patients. It was a predominantly male (75%, 75/100) study cohort, with an average age of 58.2±8.0 years. The baseline characteristics of patients who underwent EVH were similar to those patients who underwent OVH. However, there were more patients with smoking history in EVH group (86% vs. 50%, P<0.01). In addition, the patients of EVH group had a higher left ventricular ejection fraction (59.7±5.1 vs. 57.3±6.7, P=0.04).
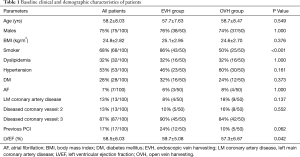
Full table
Vein harvesting variables and the continuity of SV endothelium
SV were sufficiently isolated for all scheming grafts in all the cases and no patient crossed over from EVH to OVH during the surgery. The mean harvest times of OVH (49, 41–53 minutes) group were longer than those of EVH (29, 26–35 minutes) group (P<0.01). The endothelial integrity in specimens taken before distension in EVH (81.1%±6.11%) group was similar to that of OVH (80.8%±6.58%) group (P=0.83). The results were the same after distension (EVH: 70.7%±9.73%; OVH: 68.3%±9.60%; P=0.22) (Figure 1). But a significant difference was found between the endothelium continuity before and after distending the SV (P<0.01). Even according to the harvesting method, distension still seemed to be an important factor for endothelium injury (Table 2).
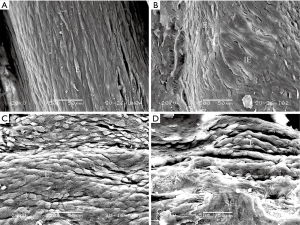

Full table
Pain assessment and wound complications
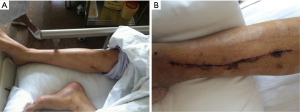
The leg pain assessed on day 7 of postoperation was significantly milder in patients of EVH group compared with patients of OVH group (1.16±0.76 vs. 2.50±0.91, P<0.01). At 3-months, the pain score at the leg wound of all the patients was no more than 1 and more patients had numbness in the OVH group (20% in OVH vs. 6.1% in EVH, P=0.04). There were 4 patients with wound complications in the OVH group. Three wound complications occurred during the first week after operation, and the other had surgical wound that was poorly healed and dehiscence 32 days after operation. Ecchymosis extending 5mm or more from the line of incision was much often seen in patients in the OVH group (26% in OVH vs. 4% in EVH, P<0.01) (Figure 2).
Follow-up results
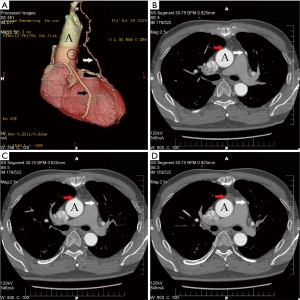
Table 3 presented the follow-up results. Ninety-nine patients (49 in the EVH group, and all 50 patients in the OVH group) accepted our questionnaire in the outpatient clinic or by telephone about 1 year after operation. Of the 99 patients, no death was found. There were no myocardial infarction (MI) or repeat revascularization reported during the follow-up period. Recurrence of angina was complained by five patients, 3 (6.1%, 3/49) in the EVH group and 2 (4%, 2/50) in the OVH group (P=0.98). There were 84 (43 in EVH and 41 in OVH) patients who took the MSCTA examination postoperation. The vein grafts of 5 (11.6%) patients in the EVH group to the left coronary artery were classified as occluded. While in the OVH group, 4 (9.8%) patients had their vein grafts to the left coronary artery occluded (Figure 3). No significant differences were found in the patency rate of vein-grafts among patients who underwent EVH and patients who underwent OVH (P=1).
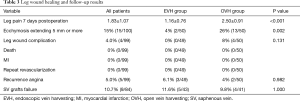
Full table
Discussion
SV still remained the most common conduits in CABG (10). But SV grafts do not have the longevity as expected, and graft failures are associated with significant adverse cardiac outcomes and mortality (11). At the same time, an open longitudinal incision along the track of the great SV is associated with relatively higher rate of complications and discomfort (12). To improve the leg-wound healing, EVH as a minimally invasive technique was introduced in CABG procedures. In 2017, the International Society for Minimally Invasive Cardiothoracic Surgery published a consensus statement recommending that EVH be the “standard of care” (class I, level B) for patients who require these conduits for coronary revascularization (13). Nowadays, according to the Society of Thoracic Surgeons National Database, EVH is used in approximately 80% of CABG patients in the United States (14).
Nevertheless, it is unclear whether EVH is independently associated with adverse clinical outcomes and vein graft failure (15). In 2009, a secondary analysis of PREVENT IV trial by Dr. Lopes et al found that EVH was associated with higher risk of MACE (16). In 2012, an observational study conducted by Dr. Williams demonstrated no association of EVH with long-term mortality or a composite of death, MI or repeat revascularization (17). Then, we aimed to investigate the relevance of EVH with clinical outcomes, graft patency and endothelial injury. In our study, the rate of MACE at one year after operation showed no difference between EVH group and OVH group. There was also no difference in the one-year patency rate of SV graft to the diagonal branch or left circumflex coronary artery between the 2 groups. More than 93% of patients can perform their daily activities (NYHA class =1, 2) without angina.
Previous studies showed that injuries of SV endothelium could trigger early graft failure (18). So, we compared endothelial injury of EVH with that of OVH. Unlike previous research (19), we found no difference between OVH and EVH in endothelial integrities with SEM. Our study also demonstrated that intraoperative distension of SV grafts could break the continuities of SV endothelium. Exposing the subendothelial tissues or media to proinflammatory and procoagulant reactions led to early SVG failure (11). Therefore, high pressure distension more likely causes early SVG failure.
EVH, as a minimally invasive technique, has been developed to reduce postoperative leg wound complications. We also assessed the complications of wound healing in the early post-surgical period. The results showed no statistical difference between the 2 groups in wound complications. But EVH group showed no serious complications. Erythema extending 5 mm or more from the line of incision was much often observed in the OVH group. At the same time, patients of EVH group had milder pain and were more satisfied with their lower limb wounds compared with patients of OVH group.
However, there are several limitations to our study. Our study enrolled only 100 patients and the follow up was completed at one year postoperation. We advised that EVH was not appropriate if SV was too superficial. It remains easy to isolate SV above the knee because of more adipose tissues. Keeping the dissection tip beside the main trunk of SV to maintain some adventitia remained very helpful to avoid injuries. So, EVH may not be suitable to every patient, especially in patients whose SVs are too superficial.
In conclusion, our study shows that EVH is not associated with worse clinic outcomes. Also found distension rather than EVH injures of the endothelium. At the same time, EVH showed good benefits in leg wound healing.
Acknowledgments
Funding: This work was supported by Beijing Science and Technology Program (China) (No. Z141107002514021) and Capital Research Funds for Application of Clinical Features Project.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-250). All authors report grants from Beijing Municipal Science and Technology Commission, during the conduct of the study.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the institutional review board and was performed in accordance with the Declaration of Helsinki and approved guidelines (Approval No.: 2015-652).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sipahi I, Akay MH, Dagdelen S, et al. Coronary artery bypass grafting vs percutaneous coronary intervention and long-term mortality and morbidity in multivessel disease: meta-analysis of randomized clinical trials of the arterial grafting and stenting era. JAMA Intern Med 2014;174:223-30. [Crossref] [PubMed]
- Gaudino M, Benedetto U, Fremes S, et al. Radial-Artery or Saphenous-Vein Grafts in Coronary-Artery Bypass Surgery. N Engl J Med 2018;378:2069-77. [Crossref] [PubMed]
- Zenati MA, Bhatt DL, Bakaeen FG, et al. Randomized Trial of Endoscopic or Open Vein-Graft Harvesting for Coronary-Artery Bypass. N Engl J Med 2019;380:132-41. [Crossref] [PubMed]
- Lichtenwalter C, de Lemos JA, Roesle M, et al. Clinical presentation and angiographic characteristics of saphenous vein graft failure after stenting: insights from the SOS (stenting of saphenous vein grafts) trial. JACC Cardiovasc Interv 2009;2:855-60. [Crossref] [PubMed]
- Zenati MA, Gaziano JM, Collins JF, et al. Choice of vein-harvest technique for coronary artery bypass grafting: rationale and design of the REGROUP trial. Clin Cardiol 2014;37:325-30. [Crossref] [PubMed]
- Wilson AP, Treasure T, Sturridge MF, et al. A scoring method (ASEPSIS) for postoperative wound infections for use in clinical trials of antibiotic prophylaxis. Lancet 1986;1:311-3. [Crossref] [PubMed]
- Revill SI, Robinson JO, Rosen M, et al. The reliability of a linear analogue for evaluating pain. Anaesthesia 1976;31:1191-8. [Crossref] [PubMed]
- Black EA, Campbell RK, Channon KM, et al. Minimally invasive vein harvesting significantly reduces pain and wound morbidity. Eur J Cardiothorac Surg 2002;22:381-6. [Crossref] [PubMed]
- Levisman JM, Budoff MJ, Karlsberg RP. Long-term coronary artery graft patency as evaluated by 64-slice coronary computed tomographic angiography. Coron Artery Dis 2011;22:521-5. [Crossref] [PubMed]
- Sousa-Uva M, Neumann FJ, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur J Cardiothorac Surg 2018;43:689-94. [PubMed]
- Greenland P, Knoll MD, Stamler J, et al. Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. JAMA 2003;290:891-7. [Crossref] [PubMed]
- Athanasiou T, Aziz O, Al-Ruzzeh S, et al. Are wound healing disturbances and length of hospital stay reduced with minimally invasive vein harvest? A meta-analysis. Eur J Cardiothorac Surg 2004;26:1015-26. [Crossref] [PubMed]
- Ferdinand FD, MacDonald JK, Balkhy HH, et al. Endoscopic Conduit Harvest in Coronary Artery Bypass Grafting Surgery: An ISMICS Systematic Review and Consensus Conference Statements. Innovations (Phila) 2017;12:301-19. [Crossref] [PubMed]
- Sastry P, Rivinius R, Harvey R, et al. The influence of endoscopic vein harvesting on outcomes after coronary bypass grafting: a meta-analysis of 267,525 patients. Eur J Cardiothorac Surg 2013;44:980-9. [Crossref] [PubMed]
- Lopes RD, Hafley GE, Allen KB, et al. Endoscopic versus open vein-graft harvesting in coronary-artery bypass surgery. N Engl J Med 2009;361:235-44. [Crossref] [PubMed]
- Williams JB, Peterson ED, Brennan JM, et al. Association between endoscopic vs open vein-graft harvesting and mortality, wound complications, and cardiovascular events in patients undergoing CABG surgery. Jama 2012;308:475-84. [Crossref] [PubMed]
- Harskamp RE, Lopes RD, Baisden CE, et al. Saphenous vein graft failure after coronary artery bypass surgery: pathophysiology, management, and future directions. Ann Surg 2013;257:824-33. [Crossref] [PubMed]
- Kopjar T, Dashwood MR. Endoscopic Versus "No-Touch" Saphenous Vein Harvesting for Coronary Artery Bypass Grafting: A Trade-Off Between Wound Healing and Graft Patency. Angiology 2016;67:121-32. [Crossref] [PubMed]
- Thatte HS, Khuri SF. The coronary artery bypass conduit: I. Intraoperative endothelial injury and its implication on graft patency. Ann Thorac Surg 2001;72:S2245-52; discussion S2267-70.


