Estimating the direct and indirect costs of lung cancer: a prospective analysis in a Greek University Pulmonary Department
Introduction
Significant efforts have been implemented and new strategies have been adopted by the European Union to prevent tobacco use. However, estimates have projected that during 2015 the prevalence of daily smokers will reach 33.5% for males and 20.1% for females. Mortality due to lung cancer (LC) is expected to continue to be very high unless substantial efforts for global tobacco control are implemented (1). LC accounted for 336,000 deaths in Europe during 2006 (2), and for 1.6 million deaths worldwide during 2012 (3). In 2014 it is expected to cause 224,210 new cases only in USA (4). Non-small cell lung cancer (NSCLC) accounts for more than 85% of all LC cases (5). Of these cases 60% is first diagnosed as metastatic or locally advanced disease (6). Annual USA direct costs of LC were estimated at $4.9 billion in 1996 (7). A variety of studies have examined the factors which influence the costs of LC patient’s management. However, there is little evidence to show whether these costs are related to response to treatment, overall survival (OS) and patient’s quality of life (QoL). Most studies aiming to estimate total costs use specific models. Unfortunately, in Greece there is no registry from public and private insurance recording the patient’s costs. This is a major issue in several developing countries, due to lack of available data and to limited use of pharmacoeconomics as it is very difficult to estimate direct costs.
The aim of this study was to estimate the direct and indirect cost of LC patients and to evaluate if these costs were related to gender, age, smoking habit, stage and histological type of disease, treatment outcome (TO), OS, cycles of chemotherapy and patient’s QoL.
Patients and methods
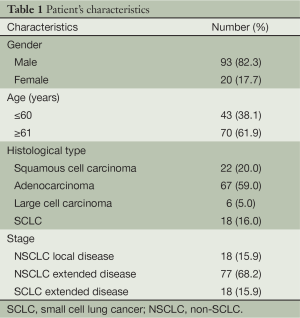
One hundred and twenty eight consecutive LC patients admitted for the first time to the Pulmonary Department of Aristotle University of Thessaloniki, G. Papanikolaou General Hospital (National Health System), Thessaloniki, Greece were initially evaluated. However, in this prospective study one hundred and thirteen patients did fulfil the inclusion criteria and were finally enrolled. Inclusion criteria were age over 18 years, initial diagnosis at the Pulmonary Department of G. Papakololaou Hospital and administration of at least one chemotherapy cycle. Patients were enrolled in this study at initial diagnosis and all data relative to diagnosis, therapy and follow-up were captured. Patients were enrolled between August 2011 and November 2011 and the follow-up was 32 months from diagnosis to the end of registry. The sample included 93 male (82%) and 20 female (18%) patients, of whom 38% was ≤60 years old and 62% was ≥61 years old.
Patients were initially arbitrarily grouped in six age categories starting from ≤40 to ≥75 years. After the first analysis it was concluded that the first three age groups (≤40, 41-50, 51-60) had similar results and this was the case also in the last three age groups (61-70, 71-75, ≥75). As a result the patients were divided in two age groups, ≤60 and ≥61 years old in the final analysis of data.
More patients were diagnosed with adenocarcinoma (59%), followed by squamous cell carcinoma (20%), small cell lung cancer (SCLC) (16%) and large cell carcinoma (5%) (Table 1).
Full table
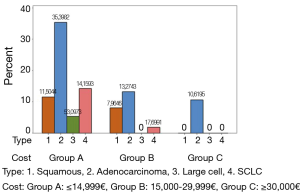
Direct cost of patients was estimated including the cost of diagnosis, hospitalisation, chemotherapy drugs, hematopoietic growth factors, chemotherapy prophylaxis drugs, imaging and laboratory tests. For the calculation of these costs the Official Government Gazette of the Hellenic Republic for years 2011, 2012, 2013 and 2014 was used (8-16). Prices and hence cost of drugs and medical procedures changed and mainly decreased among these years due to the financial condition in Greece. These changes had to be considered in these calculations. To make the analysis more efficient direct cost was divided into three groups. Group A included costs ≤€14,999, group B between €15,000-€29,999 and group C ≥€30,000.
Indirect cost was also estimated by calculating the lost days of productivity due to the disease for both patients and family caregivers. Indirect cost was estimated in days.
Finally, EORTC-QLQ-C30 (17) and LCSS (18) LC specific questionnaires were used in this study to record patient’s QoL. The questionnaires were completed every three months from diagnosis until month 21 to capture the changes in patient’s QoL.
Results
Statistical analysis
For statistical analysis software MINITAB 17.0 was used. One way anova of variance was performed using as dependent the variables response to treatment, number of chemotherapy cycles and OS, and the total direct cost as the group effect. Chi square test for goodness of fit between total direct cost and the set of variables stage and type of disease, gender and age of patients was also attempted. Indirect cost was also interpreted using all the above said variables for potential effects.
The EORTC QLQ-C30 questionnaire comprises of three categories global health status, symptom scale and functional scale (17). Each of these categories contains 2, 15 and 13 items respectively. Cronbach’s alpha was over 0.700 when reliability test was performed to justify the validity of items included in each category at each time point of questionnaire completion.
LCSS questionnaire comprises of a single score and it captured the range of symptoms though different time points (18). Cronbach’s alpha was over 0.800 when reliability test was performed to justify the validity of items included.
Diagnosis cost
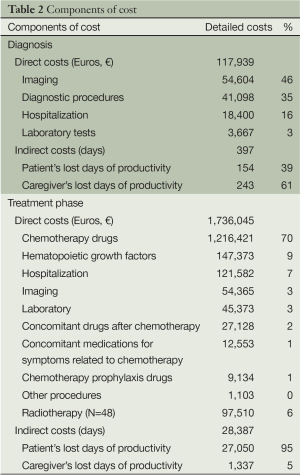
Diagnosis direct cost (of 113 patients included) was €117,939. Imaging performed for diagnostic purposes (€54,604), different other diagnostic procedures (€41,098) and hospitalization (€18,400) constituted the higher costs of diagnosis. Total direct cost of diagnosis did not differ significantly between different age groups, smokers/non-smokers, males/females, stages and histological types of disease (P>0.62). Total indirect cost was higher for patients (243 days) than for their family caregivers (154 days) and was not determined by the above factors (P>0.71). Total direct/indirect cost was not found to be related to patient’s QoL.
Treatment phase
Direct cost
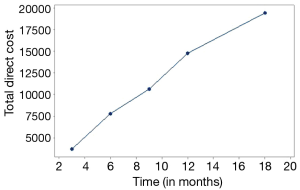
In this series of 113 patients higher total cost was due to the cost of chemotherapy drugs (€1,216,421), followed by the cost of hematopoietic growth factors (supportive care) (€147,373) and hospitalization (€85,308). As shown in Figure 1 total direct cost increased gradually through time (Figure 1).
Patients treated with radiotherapy burdened the health care system with the extra amount of €97,510 in total (Table 2).
Full table
Total direct cost was significantly related to the age and gender of patients, histological type of disease, OS and number of total chemotherapy cycles.
Younger patients appeared to burden the health care system with higher cost treatments (P=0.006), while women caused higher cost than men (P=0.012). Total direct cost differed significantly between different types of LC. As shown in Figure 2, adenocarcinoma was presented in all three cost groups (A, B, C) and increased the total patients cost significantly in comparison to other histological cancer types (P=0.003) (Figure 2).
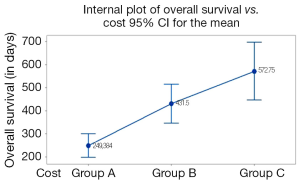
The cost of treatment rose linearly with the increase of the OS (Figure 3). As shown in Figure 3, total direct cost ≤€14,999 was associated with less days of OS (249 days) compared to total direct cost €15,000-€29,999 (431.5 days) and ≥€30,000 (572.7 days) (P<0.001). Moreover, ≥431.5 days of OS, total direct cost did not significantly differentiate because of the great overlap noticed between the 95% confidence intervals (Figure 3).
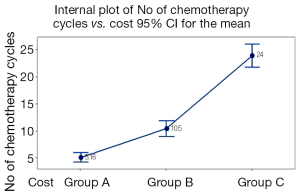
Total direct cost was significantly higher when patients received more cycles of chemotherapy (P<0.001). Total direct cost was ≤€14,999 when patients received 5.16 cycles of chemotherapy compared to €15,000-€29,999 when patients received the double number of chemotherapy cycles (10.5). As presented in Figure 4 when total direct cost reached ≥€30,000 patients received five times more chemotherapy treatments (19) (P<0.001) (Figure 4).
Total direct cost did not differ significantly between smokers and non smokers (P=0.69), stages of disease (P=0.08) and TOs (P=0.72).
Indirect cost
Total lost days of productivity were considerably higher for patients (27,050 days) than those of family caregivers (1,337 days). Total indirect cost did not differ significantly in correlation with gender, age groups, smokers and non smokers, stage and histological type of disease, TO, OS, number of chemotherapy treatment cycles (P>0.05).
Quality of life (QoL)
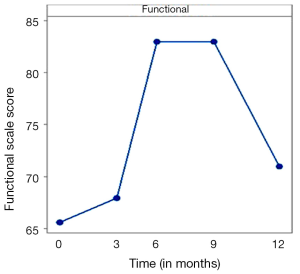
To capture patient’s QoL EORTC QLQ-C30 questionnaire was used in this study. Due to the great overlap of 95% confidence intervals among means, no statistical difference existed associated with patient’s global health status. No realistic conclusion could be drawn regarding the differences of the symptoms between different time points of questionnaire completion. The only safe conclusion was that symptoms were worse and more after three months of treatment than during diagnosis. As shown in Figure 5 in relationship to diagnosis there were three other levels of functional scale scores. Functional ability was worse after three months of treatment (68 functional scale score) than at the diagnosis time point (65.3 functional scale score). The maximum deterioration was during month 9 and 12 (83.5 functional scale score). After month 12 functional scale score was reduced and stabilized back to 72 functional scale score.
There was no significant difference of global health status score, symptoms scale score and functional scale score among different time points relative to stage and histological type of disease (P>0.05) (Figure 5).
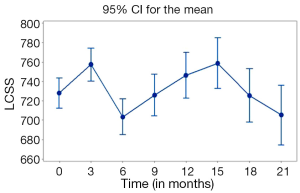
Lung Cancer Symptom Scale (LCSS) questionnaire was also completed eight times to capture the differences of patient’s symptoms at different time points. As presented in Figure 6 there was a significant improvement of symptoms between diagnosis and month 3. A steep deterioration occurred between months 3 and 6. There was a gradual improvement of symptoms between months 6 and 15, followed by a gradual deterioration until month 21 where symptom scores were similar of those in month 6 (Figure 6).
Discussion
This is one of the few prospective studies addressing direct and indirect costs of contemporary LC management and one of the very few attempts to assess these costs in Greece. Estimating the cost of resources is a very complex procedure in the Greek context. This is mainly a result of the lack of electronic capture of patient files in most hospitals in Greece and the continuous reprising of drugs and medical procedures due to the economic crisis. Moreover, in the Greek health care system prices have been established for specific diagnosis-related groups. The price charged to the patient’s insurance for a chemotherapy treatment is €80 and mainly includes most premedication drugs, consumables and procedures like blood tests, X-rays, pleural effusion drainage, blood transfusion, patients’ transportation to the hospital and physician visits (12). However, the implementation of this system creates many obstacles in the effort of pricing only the procedures performed and the resources actually used.
A detailed approach was used to estimate the direct medical costs and indirect costs. Nevertheless, the costs of hospital department infrastructure and the cost of the care received by patients outside hospital or in the emergency unit of other hospitals were difficult to account for and thus were excluded from this analysis. The indirect cost was estimated in days since there are not available data to estimate the lost productivity value of all professions of patients and caregivers included in this study.
With these limitations in mind, an estimation of the direct medical costs and indirect costs of managing LC was performed in a University Pulmonary Department, developed in a NHS Hospital, in Thessaloniki, Greece. Dedes et al. in a study performed in 2004 showed that the major part of the total cost was due to hospitalization costs. However, patients with advanced stages of LC showed the highest cost, mainly due to the costs of chemotherapy (20). Another study conducted by Braud et al. highlighted that the higher cost of treatment in patients with NSCLC was related to longer survival and duration of chemotherapy (21). In this study the cost of treatment was related to chemotherapy cycles and the use of radiotherapy was a variable which increased substantially the economic burden of LC.
The results of another study by Lanuti et al. which estimated the costs between different decades showed that the greatest single category of expense was chemotherapy (31%), followed by surgery (24%), inpatient medical (17%), radiation therapy (12%) and diagnostics (5%) (22).
These results are in consistency with the outcomes of this study. However, there are results from several countries (19,23-27) which have attributed the highest economic burden to hospitalisations rather than chemotherapy drugs. According to the results of these studies hospitalisation accounted for between 31% and 71% of total costs (25-29). In the present study hospitalisation cost was the third higher cost after chemotherapy drugs and hematopoietic growth factors but this can be explained by the significant number of patients diagnosed with adenocarcinoma (59%) and included in this study. Healthcare expenses have increased for NSCLC and especially for patients diagnosed with adenocarcinoma due to the new treatment therapies used (30), including molecular targeted drugs and third-generation chemotherapies (tyrosine-kinase inhibitors, pemetrexed which belongs in the category of chemotherapy drugs called folate antimetabolite, and angiogenesis inhibitors) (6,31,32).
A much smaller number of studies have focused on the estimation of indirect costs of LC patient management and different components have been used for these estimations like lost days of productivity, short term disability and early death (33,34). Consequently, it is difficult to make comparisons and draw out safe conclusions regarding the generalizability of indirect cost estimations.
EORT QLQ-C30 and LCSS are very commonly used in cost-effectiveness studies and economic analysis of new treatment therapies (35,36). However, the use of these questionnaires for the assessment of patient’s QoL in relation to the cost of treatment is very restricted. In the present study there was no correlation between cost of treatment and QoL and consequently it can be concluded that more expensive therapies are not always related to better QoL and less symptoms.
It is difficult to make comparison between the results from this study and others estimating direct and indirect costs due to the differences in treatment patterns, health care systems, patient cohorts, unit costs, study design and type of analysis (37).
Therefore, before making any claims on the generalizability of the results of this study, one would have to assess the different factors and patters used in each country. Further research on the economic burden of LC patient management at other cancer departments of the country would be a significant extension of this study.
Conclusions
This prospective study provides an estimation of the direct medical costs and indirect costs of LC management in a cohort of NSCLC and SCLC patients. Total direct cost was associated with the increased number of total chemotherapy cycles, longer OS, adenocarcinoma type of cancer, female gender and younger patients. The largest overall component of direct cost was the cost of chemotherapy drugs. Indirect cost was considerably higher for patients than caregivers and did not significantly differ when compared to the above factors. No significant conclusion was drawn regarding the relation between QoL and total direct/indirect cost. Further studies are needed to confirm the above results.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Strong K, Guthold R, Yang J, et al. Tobacco use in the European region. Eur J Cancer Prev 2008;17:162-8. [PubMed]
- Sichletidis TL. eds. Pulmonology, 1st Edition. Thessaloniki: University Studio Press, 2009.
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [PubMed]
- American Cancer Society. Cancer facts and figures 2014. Available online: http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf
- Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24:4539-44. [PubMed]
- Ramalingam SS, Dahlberg SE, Langer CJ, et al. Outcomes for elderly, advanced-stage non-small cell lung cancer patients treated with bevacizumab in combination with carboplatin and paclitaxel: analysis of Eastern Cooperative Oncology Group Trial 4599. J Clin Oncol 2008;26:60-5. [PubMed]
- Fox KM, Brooks JM, Kim J. Metastatic non-small cell lung cancer: costs associated with disease progression. Am J Manag Care 2008;14:565-71. [PubMed]
- Cost of medical procedures as included in the Presidential Decrees 157/1991, 163/1988, 81/1988, 138/1990, 427/1991, 38/1993, 65/1996 and 114/2000 and of those who have been costed from Central Health Council and have been accepted by the Pry minister of Health in Euros. Available online: http://isx.gr/sites/default/files/kostologimes-exetaseis.pdf
- Ministry of Health, Law 3816/2010 Complementary table of medications Law 3816/2010. Available online: http://www.moh.gov.gr/articles/times-farmakwn/deltia-timwn/646-deltia-timwn-farmakwn-2-8-2011
- Government Gazette Issue B1702/1-8-2011, Diagnosis related groups. Available online: http://www.et.gr/index.php?option=com_wrapper&view=wrapper&Itemid=104&lang=en
- Government Gazette Issue B3100/30-12-2011. Available online: http://www.et.gr/index.php?option=com_wrapper&view=wrapper&Itemid=104&lang=en
- Government Gazette Issue A41/1-3-2012. Available online: http://www.et.gr/index.php?option=com_wrapper&view=wrapper&Itemid=104&lang=en
- Government Gazette Issue B545/2012. Available online: http://www.et.gr/index.php?option=com_wrapper&view=wrapper&Itemid=104&lang=en
- Government Gazette Issue B940/ 27-3-2012, Diagnosis related Groups. Available online: http://www.et.gr/index.php?option=com_wrapper&view=wrapper&Itemid=104&lang=en
- Government Gazette Issue B64/16-1-2014. Available online: http://www.et.gr/index.php?option=com_wrapper&view=wrapper&Itemid=104&lang=en
- Government Gazette Issue 88/21-1-2014. Available online: http://www.et.gr/index.php?option=com_wrapper&view=wrapper&Itemid=104&lang=en
- Montazeri A, Gillis CR, McEwen J. Quality of life in patients with lung cancer: a review of literature from 1970 to 1995. Chest 1998;113:467-81. [PubMed]
- Hollen PJ, Gralla RJ, Kris MG, et al. Quality of life during clinical trials: conceptual model for the Lung Cancer Symptom Scale (LCSS). Support Care Cancer 1994;2:213-22. [PubMed]
- Oliver E, Killen J, Kiebert G, et al. Treatment pathways, resource use and costs in the management of small cell lung cancer. Thorax 2001;56:785-90. [PubMed]
- Dedes KJ, Szucs TD, Bodis S, et al. Management and costs of treating lung cancer patients in a university hospital. Pharmacoeconomics 2004;22:435-44. [PubMed]
- Braud AC, Lévy-Piedbois C, Piedbois P, et al. Direct treatment costs for patients with lung cancer from first recurrence to death in france. Pharmacoeconomics 2003;21:671-9. [PubMed]
- Lanuti M, Hong HJ, Ali S, et al. Observations in lung cancer over multiple decades: an analysis of outcomes and cost at a single high-volume institution. Eur J Cardiothorac Surg 2014;46:254-61; discussion 261. [PubMed]
- Kutikova L, Bowman L, Chang S, et al. The economic burden of lung cancer and the associated costs of treatment failure in the United States. Lung Cancer 2005;50:143-54. [PubMed]
- Fleming I, Monaghan P, Gavin A, et al. Factors influencing hospital costs of lung cancer patients in Northern Ireland. Eur J Health Econ 2008;9:79-86. [PubMed]
- Demeter SJ, Jacobs P, Chmielowiec C, et al. The cost of lung cancer in Alberta. Can Respir J 2007;14:81-6. [PubMed]
- Pompen M, Gok M, Novák A, et al. Direct costs associated with the disease management of patients with unresectable advanced non-small-cell lung cancer in The Netherlands. Lung Cancer 2009;64:110-6. [PubMed]
- Kang S, Koh ES, Vinod SK, et al. Cost analysis of lung cancer management in South Western Sydney. J Med Imaging Radiat Oncol 2012;56:235-41. [PubMed]
- Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 1997;111:1710-7. [PubMed]
- Zelen M. Keynote address on biostatistics and data retrieval. Cancer Chemother Rep 3 1973;4:31-42. [PubMed]
- Zeng X, Karnon J, Wang S, et al. The cost of treating advanced non-small cell lung cancer: estimates from the Chinese experience. PLoS One 2012;7:e48323. [PubMed]
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44. [PubMed]
- Landi L, Cappuzzo F. Targeted therapies: Front-line therapy in lung cancer with mutations in EGFR. Nat Rev Clin Oncol 2011;8:571-3. [PubMed]
- Chang S, Long SR, Kutikova L, et al. Estimating the cost of cancer: results on the basis of claims data analyses for cancer patients diagnosed with seven types of cancer during 1999 to 2000. J Clin Oncol 2004;22:3524-30. [PubMed]
- Weissflog D, Matthys H, Hasse J, et al. Epidemiology and costs of lung cancer in Germany. Pneumologie 2001;55:333-8. [PubMed]
- Dancey J, Shepherd FA, Gralla RJ, et al. Quality of life assessment of second-line docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy: results of a prospective, randomized phase III trial. Lung Cancer 2004;43:183-94. [PubMed]
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76. [PubMed]
- Navaratnam S, Kliewer EV, Butler J, et al. Population-based patterns and cost of management of metastatic non-small cell lung cancer after completion of chemotherapy until death. Lung Cancer 2010;70:110-5. [PubMed]