
Changing paradigm in advanced and metastatic non-small cell lung cancer
Background
Approximately 18% of all cancer-associated mortality is related to lung cancer (1). More than 80% of all lung cancer cases are due to non-small cell lung cancer (NSCLC) (2). Surgery in the form of lobectomy and mediastinal lymph node dissection remains the primary modality of treatment for patients who have resectable diseases (stage I–IIIA). Surgery is often followed by cisplatin-based adjuvant platinum doublet (stage ≥ IIA, American Joint Committee on Cancer 8th edition). The cisplatin-based chemotherapy offers an additional 5% 5-year survival compared to surgery alone (3,4). However, majority of patients with NSCLC have unresectable disease (either stage III or IV) at the time of diagnosis (5). Management of this patient population has changed significantly in recent years, which requires nuanced and patient-specific management. Herein, we will discuss the management of this patient population with particular emphasis on recent updates.
Management of stage III NSCLC
A critical distinction that helps narrow the treatment algorithm is determining whether the disease is resectable. Although tumor resectability greatly depends on dimension and location, the most important factor is the extent of regional lymph nodes involvement.
Resectable versus unresectable disease
N1 disease is typically a resectable disease. The management usually involves surgery, if technically feasible. These patients will benefit from cisplatin-based chemotherapy post-surgery. N3 disease is treated with definitive chemo-radiation and surgery is not recommended. The management of N2 disease, which involves ipsilateral mediastinal or subcarinal lymph nodes, is greatly controversial and the indication for surgery in this subset of patients is not well defined. Patients with clinical N2 disease (radiological or mediastinal staging) are usually offered chemo-radiation therapy concurrently. Surgery can be offered to a small subset of patients with one-station, non-bulky N2 (not clearly defined) nodal involvement although there is lack of prospective data for this approach. We should be very careful while selecting patients for tri-modality treatment in this setting because of significant early treatment-related mortality. In an INT0139 intergroup trial, 429 patients with pN2 disease (T1, T2 or T3 primary tumor) and technically resectable tumor were randomized either to receive concurrent chemo and radiation therapy followed by surgical resection (n=216) or concurrent chemo and radiation therapy followed by completion of definitive-dose of radiation therapy (n=213) (6). Progression-free survival (PFS) was much better in the surgery group (median PFS 12.8 versus 10.5 months) but there was no significant difference in median overall survival (OS).
Adjuvant versus neoadjuvant therapy
In resectable disease, following resection, cisplatin-based platinum-doublet is the treatment of choice. The goal of adjuvant chemotherapy is to eliminate distant micro metastases and prevent disease recurrence and hence improve the OS. The Lung Adjuvant Cisplatin Evaluation (LACE) performed a meta-analysis of 4,584 patients that analyzed five large trials of post-surgical adjuvant chemotherapy. The study showed a 5.4% OS benefit at 5 years with post-surgical chemotherapy compared to no treatment after surgery (4). Three major trials analyzed by LACE were the Adjuvant Lung Project Italy (ALPI), International Adjuvant Lung Cancer Trial (IALT), and Adjuvant Navelbine International Trialist Association (ANITA) Trial. ALPI was the first reported trial comparing cisplatin-based chemotherapy versus observation (7). More than 1,200 patients with stage I–IIIA were enrolled in the study. In this study, three-drug regimen chemotherapy (MVP—mitomycin/vindesine/cisplatin) was compared to observation. The results showed no survival difference (OS and PFS) between the two arms. IALT enrolled 1,867 patients with resected, stage I–IIIA disease and randomized patients into either chemotherapy or observation group (8). The chemotherapy arm consisted of 3 to 4 courses of cisplatin-containing chemo regimen. The study found significantly higher OS and PFS at 5 years in chemotherapy group. Likewise, ANITA Trial randomized 849 patients to either chemotherapy with treatment consisting of cisplatin and vinorelbine or observation (9). This trial also showed a survival benefit with the post-surgical chemotherapy.
Neoadjuvant chemotherapy, which is an alternative approach, is given before the surgery, and may facilitate tumor size reduction rendering surgery easier in this subset of patients. In a randomized trial of 60 patients with stage IIIA (N2) disease who either had pre- or post-operative chemotherapy combination along with surgery versus surgery alone, the median OS was 64 months for surgery plus chemotherapy group versus 11 months for surgery only group (10). In another study, patients were randomized to neoadjuvant cisplatin-gemcitabine plus surgery and surgery only. A significant 3-year PFS survival advantage was noted in combination arm for stage IIB/IIIA disease (55.4% versus 36.1%; P=0.002) (11).
Additionally, a systematic review of 32 randomized studies comprising of more than 10,000 patients showed no difference in survival between neoadjuvant and adjuvant therapies (12). Given the concern for delay in potentially curative surgery from drug toxicity and disease progression on chemotherapy making the disease unresectable later, adjuvant rather than neoadjuvant chemotherapy is the preferred approach. Various neoadjuvant trials are ongoing utilizing immunotherapy or chemo-immunotherapy to estimate the benefit of immunotherapy and/or chemo-immunotherapy in the neoadjuvant setting (NCT 03425643, NCT 02998528, NCT 02716038, NCT 03456063, NCT 03800134, NCT 02818920, and NCT 02994576).
Concurrent chemo-radiation therapy
For patients that have unresectable locally advanced disease (most N2 and all N3 cases), concurrent chemo-radiation followed by durvalumab is now recommended. Concurrent chemo-radiation showed statistically significant 5-year survival benefit over sequential chemo-radiation in a randomized phase-3, RTOG 9410 study, with a survival rate of 10% in sequential therapy versus 16% in once-daily thoracic radiation with concurrent chemotherapy and 13% in twice-daily radiation with concurrent chemotherapy (13). However, non-hematological acute toxicities including esophagitis and pneumonitis were higher in the concurrent chemo-radiation arm. However, even with this intense treatment, prognosis continued to remain poor with median PFS of about 8 months and 5-year OS rate of 15%.
Most recently, PACIFIC trial studied benefit of maintenance durvalumab in unresectable NSCLC patients following chemo-radiation. In this phase-3 trial, 709 patients with unresectable disease after receiving definitive chemo- and radiation therapy concurrently were randomized to receive 1 year of maintenance durvalumab every 2 weeks or placebo (14). The study showed significant survival benefit with the maintenance immunotherapy (both PFS and OS) (15). Durvalumab is therefore now recommended to all patients who do not have disease progression following concurrent chemo and radiation based on the result of this study.
Management of locally advanced or metastatic NSCLC
Historical perspective
About 60% to 70% of lung cancer cases will have metastatic disease at the time of presentation (2,16). Initially felt to be toxic and ineffective, chemotherapy has been shown by various trials to engender survival benefit (17-19). Additionally, chemotherapy reduces symptoms and maintains good quality of life. Chemotherapy for NSCLC traditionally has been platinum-based two-drug regimen called “platinum doublet”. Because of survival benefit, for the past two decades, patients with metastatic lung disease were offered platinum-doublet chemotherapy. An initial landmark trial conducted by Schiller and colleagues showed a response rate of 20% and a median OS of 8 months with platinum-doublet chemotherapy (20). Further successful phase III studies have shown a response rate with the platinum-doublet chemotherapy of about 30%; median PFS at best of about 6 months and median OS of about 12 months (21-23).
Almost all patients will eventually progress on platinum-doublet chemotherapy. Following disease progression, patients are often treated with docetaxel based on the result of the TAX-320 study. In this trial, 373 patients received D100 (100 mg/m2) or D75 (docetaxel 75 mg/m2) every 3 weeks or V/I (vinorelbine or ifosfamide) (24). This trial demonstrated that D75 was superior to V/I and had favorable safety profile when compared with D100. Docetaxel with ramucirumab is an alternative based on the REVEL study (25). In this multi-center study involving more than 1,200 NSCLC cases, patients either received docetaxel and placebo (n=625) or docetaxel and ramucirumab (n=628). The study demonstrated a modest improvement in OS in the ramucirumab group (median OS: 10.5 versus 9.1 months). In this study, ramucirumab arm had more neutropenia with or without fever, hypertension, and bleeding. Since there was modest survival benefit with increased toxicity, ramucirumab-docetaxel combination should be avoided in elderly population with marginal performance status.
Introduction of immunotherapy in second line setting
CheckMate-017 was a phase-3 trial which randomized patients with squamous cell lung cancer into either nivolumab or docetaxel (26). The study showed significant survival benefit with nivolumab (median OS: 9.2 versus 6.0 months). Likewise, CheckMate-057 showed survival benefit with nivolumab in patients with non-squamous NSCLC (27). Long follow-up data from these two studies confirm durable benefit from nivolumab (28,29). Subsequently, pembrolizumab demonstrated OS benefit over docetaxel for those with PD-L1 ≥1% (30). Likewise, atezolizumab also prolonged OS over docetaxel (31). Based on the results of these trials, immunotherapy is recommended for those patients who have disease progression on platinum-doublet chemotherapy.
Immunotherapy in the first-line setting in patients without sensitizing mutation
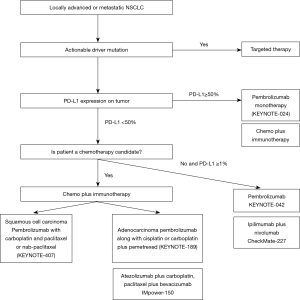
KEYNOTE-024 was a phase-3 study where patients with metastatic NSCLC and with tumor proportion score of ≥50%, received either pembrolizumab monotherapy for up to 35 cycles or platinum-doublet standard chemotherapy for 4 to 6 cycles (32). The study demonstrated superior 6-month OS with pembrolizumab monotherapy over standard chemotherapy (80.2%; 95% CI, 72.9–85.7% versus 72.4%; 95% CI, 64.5–78.9%). In addition, the response rate and PFS were superior in the immunotherapy arm. Side effect profile was also favorable with pembrolizumab monotherapy. Pembrolizumab monotherapy is therefore a favored front-line treatment option for those with stage IV NSCLC without sensitizing mutation and with tumor proportion score of ≥50%. An updated analysis of KEYNOTE-024 showed a median OS of 30 months for those who received pembrolizumab versus 14.2 months for those who received chemotherapy (33). However, we have to realize that less than one-third of patients will have PD-L1 ≥50%.
CheckMate-026 trial randomized patients with PD-L1 ≥1% to either receive nivolumab or standard platinum-doublet (34). For patients with PD-L1 ≥5%, response rate was 26% versus 33% and median PFS was 4.2 versus 5.9 months in immunotherapy group and chemotherapy group respectively; OS was similar between the two groups. Based on this result, nivolumab monotherapy has not been approved by regulatory bodies in the first-line setting.
KEYNOTE-042 trial randomized treatment naïve patients with PD-L1 ≥1% to pembrolizumab versus standard chemotherapy. The trial aimed to compare OS between two arms in three subsets of patients based on the PD-L1 expression (≥50%, ≥20%, and ≥1%). It revealed survival benefit in all three subgroups with pembrolizumab treatment. However, no OS difference was noted for PD-L1 ≥1% to 49%, suggesting no superior efficacy of pembrolizumab over chemotherapy for PD-L1 ≥1% to 49%. Pembrolizumab monotherapy could be recommended in a subset of cases with PD-L1 <49% but ≥1% who may not be a good candidate to receive chemotherapy or chemo-immunotherapy (35).
Chemo-immunotherapy in the first line setting
KEYNOTE-189 randomized patients with stage IV non-squamous histology without sensitizing mutation into platinum-pemetrexed-pembrolizumab induction followed by pembrolizumab-pemetrexed maintenance or platinum-pemetrexed induction followed by pemetrexed maintenance (36). It revealed significant improvement in PFS and OS with chemo-immunotherapy (OS at 12 months was 69.2% versus 49.4%, median PFS was 8.8 versus 4.9 months). The survival benefit was also seen for PD-L1 <1%. Additionally, KEYNOTE-407 demonstrated PFS and OS benefit in patients with squamous NSCLC when pembrolizumab was added with carboplatin-paclitaxel (37).
Combination chemo-immunotherapy is now the front-line treatment regimen for stage IV NSCLC patients without driver mutation. Notably, for those with PD-L1 ≥50%, pembrolizumab monotherapy is a favored treatment option as mentioned above. Combination chemo-immunotherapy should be utilized in symptomatic patients with high volume disease, where a rapid and robust response is desired.
Additionally, in IMPower 130 study, patients were randomized either to platinum-doublet (carboplatin every 3 weeks plus nab-paclitaxel every week) or to receive chemotherapy plus atezolizumab for 4–6 cycles (38). Patients on atezolizumab arm were placed on atezolizumab maintenance. Patients on chemotherapy-only arm were either provided best supportive care or were allowed to have switch maintenance to pemetrexed. PFS and OS increased significantly with the addition of atezolizumab. This regimen can potentially be used in this subset of patient but will need a weekly clinic visit for nab-paclitaxel infusion in the initial induction phase which may not be convenient to our patient population.
Chemo-immunotherapy in conjunction with vascular endothelial growth factor inhibitor
In the IMPower 150 study, non-squamous cases were randomized either to receive platinum-doublet/atezolizumab (ACP), platinum-doublet/bevacizumab (BCP) or platinum-doublet/bevacizumab/atezolizumab (ABCP) combinations (39). After induction treatment of 4 to 6 cycles, these patients were then given atezolizumab, bevacizumab, or both. The platinum-doublet/bevacizumab/atezolizumab showed longer PFS and OS as compared to the chemotherapy-bevacizumab combination.
Of note, a separate report of this trial was published for EGFR-mutant cases, who either had disease progression or had intolerance to standard tyrosine kinase inhibitor (TKI) (40). The report demonstrated that in the subset of patients with sensitizing EGFR mutation, the ABCP regimen showed better OS compared with BCP regimen. ABCP regimen could potentially be utilized in those with sensitizing EGFR mutation that have disease progression on standard TKI or are intolerant to TKI treatment. More data are necessary to further delineate the role of bevacizumab and the ideal chemotherapy backbone for EGFR-mutant cases managed with chemo-immunotherapy. For patients who are not candidates for bevacizumab or a taxane, it is the opinion of the authors that the KEYNOTE-189 regimen is a reasonable option.
Combination of immune check point inhibitors
CheckMate-227 randomized patients without sensitizing mutations into three arms: nivolumab-ipilimumab combination, nivolumab, and standard platinum-doublet (41,42). The study demonstrated significantly longer PFS with the immunotherapy combination in those with high tumor mutation burden (defined as tumor mutational burden ≥10 mutations per megabase) (41). Second part of the study was published later and demonstrated that immunotherapy combination showed an improvement in OS when compared with standard platinum-doublet for those with PD-L1 ≥1% (42). Ipilimumab-nivolumab combination could be used in certain clinical setting when chemotherapy is contraindicated. Table 1 provides the summary of various phase III studies utilizing immunotherapy in advanced or metastatic NSCLC in the first-line setting.

Full table
Targeted therapy
In comparison to standard chemotherapy, EGFR TKIs not only improved survival but also improved health-related quality of life (43,44). Osimertinib was initially approved for those with a resistant T790M mutation following first-line EGFR-TKI (erlotinib or gefitinib) treatment (45). Osimertinib is now a preferred front-line therapy in sensitizing EGFR-mutant cases based on the FLAURA study (46). PFS and OS were significantly higher in osimertinib arm when compared with the standard EGFR-TKI group (46,47). Also, the rates of central nervous system (CNS) progression were substantially lower in osimertinib group.
In an open-label ALEX study, ALK-positive NSCLC patients were randomized either to oral alectinib or crizotinib (48). The study demonstrated significantly higher disease progression or deaths in crizotinib group when compared with alectinib (68% versus 41%). Also, 12-month PFS was substantially higher in alectinib group. Additionally, rate of CNS progression was substantially lower in the alectinib arm (45% versus 12%). J-ALEX was a similar study conducted in the Japanese population with a lower dose of alectinib (49). The study demonstrated favorable safety profile and better PFS with oral alectinib compared to crizotinib. Based on the result of these trials, oral alectinib is now the favored frontline treatment regimen for ALK mutant cases. In ALTA-1L trial, ALK-positive cases were randomized to brigatinib or crizotinib (50). One-year PFS was considerably higher in brigatinib group (67% versus 43%). Although brigatinib is an alternative, alectinib is still preferable first line option given long-term follow-up data.
Shaw and colleagues in their open-label study treated 53 patients with ROS1 rearrangement with oral crizotinib (51). Seventy two percent of cases achieved an objective response which lasted for a median duration [duration of response (DOR)] of 24.7 months. Likewise, the median PFS was 19.3 months and median OS was 51.4 months (51,52). Entrectinib is a CNS-penetrant TKI and has activity against ROS1-positive NSCLC. An integrated analysis of three ongoing early phase trials with entrectinib treatment on ROS-1 positive NSCLC showed an objective response rate (ORR) of 77%, DOR of 24.6 months (53). Both of these drugs are approved by regulatory bodies for the treatment of NSCLC with ROS1 rearrangement. We recommend using entrectinib over crizotinib for patients with known CNS disease.
In a phase-2 study of BRAF (V600E) mutant NSCLC patients, combination of BRAF inhibition with MEK inhibition (dabrafenib plus trametinib) showed an ORR of 63.2% and median PFS of 9.7 months (54,55). Dabarafenib plus trametinib is now the front-line regimen for those with BRAF mutation. A single agent dabrafenib or vemurafenib can be offered for those who are not tolerating the combination therapy (BRAF inhibition and MEK inhibition) but the response rate is about 30–40% (54,56).
In phase 1–2 trial involving pediatric and adult patients with NTRK fusion positive cancers, larotrectinib treatment yielded an ORR of 75% (independent review) and at a median follow-up of 9.4 months, 86% of cases were responding to treatment (57). Likewise, an integrated analysis of three ongoing early phase trials with entrectinib treatment on NTRK fusion positive cancers showed an ORR of 57% and median DOR of 10 months. Both, larotrectinib and entrectinib can therefore be used in patients with NTRK gene fusion positive NSCLC (58).
Conclusions
In summary, disease stage remains the primary factor that guides management in NSCLC. For stage III disease, multidisciplinary approach is recommended. For resectable disease, the standard of care is resection followed by chemotherapy. For unresectable disease, platinum-based chemo and radiation given concurrently followed by maintenance durvalumab is recommended. In more advanced disease, treatment is individualized depending on the mutational profile and the PD-L1 expression (Figure 1). Patients with sensitizing mutation are recommended to have personalized mutation directed treatment. For those without any sensitizing mutation, combination of chemo-immunotherapy is recommended. A small fraction of these cases that lack sensitizing mutation but have PD-L1 expression of ≥50%, pembrolizumab monotherapy is favored over chemo-immunotherapy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Chi Wan Koo) for the series “Contemporary Practice in Thoracic Neoplasm Diagnosis, Evaluation and Treatment” published in Journal of Thoracic Disease. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1472). The series “Contemporary Practice in Thoracic Neoplasm Diagnosis, Evaluation and Treatment” was commissioned by the editorial office without any funding or sponsorship. Dr. KP reports personal fees from Astra-Zeneca. Dr. KL reports honoraria from Onc Live and Takeda. Dr. AD reports personal fees from Roche/Genentech, personal fees from OncLive. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584-94. [Crossref] [PubMed]
- Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004;350:351-60. [Crossref] [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [Crossref] [PubMed]
- Lu T, Yang X, Huang Y, et al. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag Res 2019;11:943-53. [Crossref] [PubMed]
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379-86. [Crossref] [PubMed]
- Scagliotti GV, Fossati R, Torri V, et al. Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA non-small-cell Lung cancer. J Natl Cancer Inst 2003;95:1453-61. [Crossref] [PubMed]
- Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 2005;352:2589-97. [Crossref] [PubMed]
- Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 2006;7:719-27. [Crossref] [PubMed]
- Roth JA, Fossella F, Komaki R, et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. J Natl Cancer Inst 1994;86:673-80. [Crossref] [PubMed]
- Scagliotti GV, Pastorino U, Vansteenkiste JF, et al. Randomized phase III study of surgery alone or surgery plus preoperative cisplatin and gemcitabine in stages IB to IIIA non-small-cell lung cancer. J Clin Oncol 2012;30:172-8. [Crossref] [PubMed]
- Lim E, Harris G, Patel A, et al. Preoperative versus postoperative chemotherapy in patients with resectable non-small cell lung cancer: systematic review and indirect comparison meta-analysis of randomized trials. J Thorac Oncol 2009;4:1380-8. [Crossref] [PubMed]
- Curran WJ Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011;103:1452-60. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379:2342-50. [Crossref] [PubMed]
- National Cancer Institute Surveillance E, and End Results Program. Cancer Stat Facts: Lung and Bronchus. Assessed on February 27, 2020. Available online: at https://seer.cancer.gov/statfacts/html/lungb.html
- Cullen MH, Billingham LJ, Woodroffe CM, et al. Mitomycin, ifosfamide, and cisplatin in unresectable non-small-cell lung cancer: effects on survival and quality of life. J Clin Oncol 1999;17:3188-94. [Crossref] [PubMed]
- Rapp E, Pater JL, Willan A, et al. Chemotherapy can prolong survival in patients with advanced non-small-cell lung cancer--report of a Canadian multicenter randomized trial. J Clin Oncol 1988;6:633-41. [Crossref] [PubMed]
- Marino P, Pampallona S, Preatoni A, et al. Chemotherapy vs supportive care in advanced non-small-cell lung cancer. Results of a meta-analysis of the literature. Chest 1994;106:861-5. [Crossref] [PubMed]
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92-8. [Crossref] [PubMed]
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51. [Crossref] [PubMed]
- Paz-Ares LG, de Marinis F, Dediu M, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol 2013;31:2895-902. [Crossref] [PubMed]
- Patel JD, Socinski MA, Garon EB, et al. PointBreak: a randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. J Clin Oncol 2013;31:4349-57. [Crossref] [PubMed]
- Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol 2000;18:2354-62. [Crossref] [PubMed]
- Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014;384:665-73. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Antonia SJ, Borghaei H, Ramalingam SS, et al. Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: a pooled analysis. Lancet Oncol 2019;20:1395-408. [Crossref] [PubMed]
- Gettinger S, Horn L, Jackman D, et al. Five-Year Follow-Up of Nivolumab in Previously Treated Advanced Non-Small-Cell Lung Cancer: Results From the CA209-003 Study. J Clin Oncol 2018;36:1675-84. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol 2019;37:537-46. [Crossref] [PubMed]
- Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2415-26. [Crossref] [PubMed]
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819-30. [Crossref] [PubMed]
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019;20:924-37. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Reck M, Mok TSK, Nishio M, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med 2019;7:387-401. [Crossref] [PubMed]
- Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med 2018;378:2093-104. [Crossref] [PubMed]
- Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2019;381:2020-31. [Crossref] [PubMed]
- Thongprasert S, Duffield E, Saijo N, et al. Health-related quality-of-life in a randomized phase III first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients from Asia with advanced NSCLC (IPASS). J Thorac Oncol 2011;6:1872-80. [Crossref] [PubMed]
- Yang JC, Hirsh V, Schuler M, et al. Symptom control and quality of life in LUX-Lung 3: a phase III study of afatinib or cisplatin/pemetrexed in patients with advanced lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3342-50. [Crossref] [PubMed]
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med 2020;382:41-50. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet 2017;390:29-39. [Crossref] [PubMed]
- Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2027-39. [Crossref] [PubMed]
- Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963-71. [Crossref] [PubMed]
- Shaw AT, Riely GJ, Bang YJ, et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Ann Oncol 2019;30:1121-6. [Crossref] [PubMed]
- Drilon A, Siena S, Dziadziuszko R, et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1-2 trials. Lancet Oncol 2020;21:261-70. [Crossref] [PubMed]
- Planchard D, Besse B, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol 2016;17:984-93. [Crossref] [PubMed]
- Planchard D, Besse B, Groen HJM, et al. An open-label phase II trial of dabrafenib (D) in combination with trametinib (T) in patients (pts) with previously treated BRAF V600E–mutant advanced non-small cell lung cancer (NSCLC; BRF113928). J Clin Oncol 2016;34:107. [Crossref]
- Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N Engl J Med 2015;373:726-36. [Crossref] [PubMed]
- Drilon A, Laetsch TW, Kummar S, et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med 2018;378:731-9. [Crossref] [PubMed]
- Doebele RC, Drilon A, Paz-Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol 2020;21:271-82. [Crossref] [PubMed]


