
The insidious presentation and challenging management of esophageal perforation following diagnostic and therapeutic interventions
Introduction
Perforation of the esophagus can be defined as a transmural disruption of its continuity, which results in leakage of intraluminal contents into the surrounding tissues. Most esophageal perforations are caused by diagnostic and therapeutic interventions, followed by spontaneous rupture, foreign body ingestion, trauma and malignancy (1-3). Since its first description by Hermann Boerhaave nearly 300 years ago (4), esophageal perforation remains a potentially life-threatening condition. The mortality depends on the etiology, part of the esophagus involved, presence of underlying pathology and time elapsed from symptom onset to diagnosis. Indeed, the reported mortality ranges from 10% to 25% when therapy is instigated within 24 hours but increases up to 60% when treatment is delayed beyond 48 hours (5). Unfortunately, the rarity of this pathological condition and its nonspecific presentation can lead to delay in diagnosis in more than 50% of patients (6). In these cases, the optimal therapy remains unclear. It becomes evident that esophageal perforation continues to present diagnostic and therapeutic challenges. Therefore, clinicians must be aware of its potentially insidious presentation and knowledgeable regarding the management options of this highly morbid condition. The present article reviews solely iatrogenic esophageal perforations and aims to identify their incidence, aid their diagnosis and elucidate the controversial aspects of their treatment. This review does not include leaks from esophageal anastomoses, which represent a different clinical entity.
Etiology
Diagnostic and therapeutic interventions on the esophagus or adjacent organs are the leading cause of esophageal perforation, accounting for 46.5% of all cases in a systematic review of 40 studies that included 1,933 patients (7). Of these interventions, esophageal instrumentation is the commonest cause of perforation. When therapeutic procedures are performed at the time of diagnostic esophageal endoscopy, the risk of perforation increases even further. For instance, the estimated risk of perforation during diagnostic esophagoscopy is as little as 0.03% and 0.11% with flexible and rigid scope respectively (8,9). This risk, however, increases significantly after argon plasma coagulation of Barrett’s esophagus, photodynamic therapy for palliation of esophageal cancer, stent placement for malignant dysphagia or stricture dilation (10-13). The risk is even higher after endoscopic mucosal resection or submucosal dissection, endoscopic variceal sclerotherapy, Nd:YAG laser therapy for palliation of esophageal cancer or pneumatic dilation for achalasia (11,14-21).
Intraoperative esophageal perforation accounts for nearly 2% of all cases and can occur at the time of neck, thoracic or abdominal surgery. Surgical procedures with risk of esophageal perforation include resection of lung cancer, pneumonectomy, pulmonary transplantation, mediastinoscopy, excision of mediastinal tumors, thoracic aortic aneurysm repair, left atrial radiofrequency ablation, hiatal hernia repair, antireflux surgery, vagotomy, thyroidectomy and operations on the cervical spine (22-30). In particular, the incidence of esophageal perforation after anterior osteosynthesis for cervical spine fracture can be as high as 3.4% (31). Lastly, esophageal perforation can be caused by various other procedures, including transesophageal echocardiography, endotracheal intubation, mini tracheostomy, transtracheal jet ventilation, Sengstaken-Blakemore or Minnesota tube placement, nasogastric tube insertion, bronchial artery embolization and radiotherapy (32-37). Table 1 summarizes the commonest causes of iatrogenic esophageal perforation.

Full table
Clinical presentation
The clinical presentation of esophageal perforation is nonspecific and can mimic that of other, commoner disorders, such as pneumonia, angina, peptic ulcer disease and pancreatitis. Typical symptoms include pain in the neck, chest, back or epigastrium, as well as dysphagia, odynophagia, dysphonia and dyspnea (6,23,25,38-40). Common clinical signs include subcutaneous emphysema, fever, tachypnoea, tachycardia and hypotension (6,22-25,38-42). Any combination of the above signs and symptoms following instrumentation of the esophagus or surgery on neighboring organs should raise the suspicion of esophageal perforation.
The symptomatology mostly depends on the time interval from the iatrogenic injury to the diagnosis, as well as the site of the perforation. Cervical esophageal perforation presents with neck pain and stiffness, dysphagia, dysphonia and bloody regurgitation. Due to attachment of the esophagus to the prevertebral fascia, the spread of oropharyngeal soilage is limited, resulting in less severe clinical manifestations compared to thoracic and abdominal perforations. Thoracic esophageal perforation causes contamination of the mediastinum, which may extend into the pleural cavities, thereby leading to pleuritic, retrosternal or interscapular pain, odynophagia, dyspnea and cough. However, this clinical presentation may be less pronounced in the presence of an intercostal chest drain that has been inserted in the pleural cavity as part of a thoracic surgical procedure. Finally, abdominal esophageal perforation contaminates the peritoneal cavity and manifests with abdominal pain, nausea and vomiting. Abdominal pain may radiate to the back if there is collection in the lesser sac or may be referred to the shoulders due to diaphragmic irritation.
Diagnostic investigations
A high level of suspicion is crucial for prompt diagnosis of iatrogenic esophageal perforation because early signs and symptoms may be subtle and misleading. Indeed, it is estimated that up to 50% of patients with esophageal perforation present with atypical clinical features leading to diagnostic delay (43).
Esophageal perforation can initially be suspected with plain radiography. In perforation of the cervical esophagus, plain neck imaging may demonstrate subcutaneous emphysema, anterior displacement of the trachea and gas in the prevertebral fascial planes on lateral view (38). In thoracic esophageal perforation, a chest radiograph may demonstrate subcutaneous emphysema, pneumomediastinum, mediastinal air-fluid level, mediastinal widening, pleural effusion, pneumothorax or hydropneumothorax (44,45). Abnormal chest radiograph is developed within 12 hours of instrumental esophageal perforation in as many as 75% of patients (46). However, these radiological findings are non-specific after intrathoracic surgical procedures and become subtler if an intercostal chest drain is in situ. In abdominal esophageal perforation, a chest radiograph may show subdiaphragmatic air.
Once esophageal perforation is suspected, contrast esophagography should promptly be performed to confirm the presence and demonstrate the site of the perforation. Iodinated water-soluble contrast agents, such as diatrizoate, have been widely recommended for the detection of esophageal perforation (47). However, the rapid transit of thin contrast media may yield negative results, especially in perforations of the upper esophagus (38). In these cases, contrast esophagography with dilute barium sulphate may be considered (6). This imaging technique can determine the precise location of the perforation and indicate whether it is confined to the mediastinum or freely communicates with the pleural or peritoneal cavities (5,47). Nevertheless, the use of barium sulphate, especially in high concentrations, can cause inflammatory response, most notably mediastinitis, and may interfere with the interpretation of subsequent imaging studies.
Contrast-enhanced computed tomography of the chest is a valuable investigation for confirming esophageal perforation and ruling out alternative diagnoses. Computed tomography is indispensable when contrast esophagography cannot be undertaken or is negative and the clinical suspicion for perforation is high (48,49). Moreover, computed tomography can provide important information for the subsequent management strategy and operative planning. Abnormal radiological findings include extraluminal contrast, mediastinal air, periesophageal fluid collection, pleural effusion, esophageal thickening and communication of the air-filled esophagus with a contiguous mediastinal air-fluid collection (49,50).
Flexible esophagoscopy allows direct visualization of the perforation. However, esophageal endoscopy is not recommended as primary diagnostic procedure because air insufflation can cause further dissection of the perforation (51). For the same reason, if there is suspicion of perforation during an endoscopic procedure, meticulous inspection of the esophagus should be undertaken without air insufflation prior to removal of the endoscope.
Pleural fluid analysis can confirm the diagnosis of esophageal perforation by revealing elevated salivary amylase, pH less than 6 or the presence of undigested food or liquids (52). Table 2 summarizes the key diagnostic findings in iatrogenic esophageal perforation.

Full table
Treatment
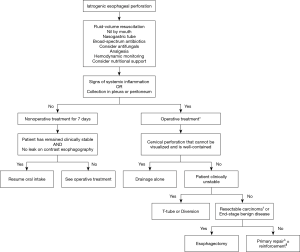
The treatment of iatrogenic esophageal perforation is mainly determined by the site and extent of the injury. Additional factors include the time interval between perforation and treatment initiation, damage to surrounding tissues, overall condition of the patient and presence of concomitant esophageal pathology (53-55). A high incidence of underlying esophageal disease has been reported and represents a marker for the iatrogenic nature of esophageal perforation (3,25). The main goals of treatment are prevention of further contamination, eradication of the infection, establishment of nutritional support and restoration of the continuity of the gastrointestinal tract. Once the diagnosis is confirmed, treatment should be commenced immediately. The patient must be kept nil per os and a nasogastric tube should be inserted to clear gastric contents and limit further contamination. Due to the lack of randomized clinical trials, an empiric regime of intravenous broad-spectrum antibiotics should be initiated as early as possible. Antifungal coverage is warranted in patients who have been hospitalized or received broad-spectrum antimicrobial agents prior to the perforation, patients on long-term antacid therapy, patients who have received steroids or other immunosuppressive therapy, patients with HIV infection and patients who fail to improve after several days of appropriate antibacterial therapy. Adequate analgesia should be provided to control pain or discomfort, but narcotic analgesics should be used cautiously in hypotensive patients. Total parenteral nutrition should be instigated if a prolonged fasting period is anticipated, while percutaneous endoscopic gastrostomy may also be considered. The patients may be transferred to a higher level of care for hemodynamic monitoring, fluid-volume resuscitation and stabilization as required. However, these preparations should not delay surgical assessment and management.
Surgical management includes drainage alone, primary closure, esophageal resection, T-tube placement, as well as exclusion and diversion techniques. The selection of surgical approach must be based on the site of the perforation. Cervical esophageal perforations that cannot be visualized and are well-contained down to the level of the carina can be managed with drainage alone through a cervical incision (3). Optimal surgical approach to perforations of the middle and lower third of the esophagus is achieved via a right thoracotomy in the sixth intercostal space and a left thoracotomy in the seventh intercostal space respectively. However, primary repair of esophageal perforation following instrumentation has also been performed successfully with video-assisted thoracoscopic surgery (56,57). Perforations of the abdominal esophagus are best approached via an upper midline laparotomy.
Primary repair has been traditionally advocated as the treatment of choice for esophageal perforations diagnosed within the first 24 hours (23,25,39,40,52). However, an increasing number of studies have identified high rates of delayed diagnosis and demonstrated improved survival rates with primary repair undertaken after the first 24 hours (3,58-60). Successful primary repair mandates debridement of necrotic tissue, drainage of the contaminated area, as well as full exposure and secure closure of the damaged mucosa to avoid leakage (58,59,61). The problem of leakage from the primary repair site led to the development of reinforcement techniques with various vascularized pedicle flaps, including parietal pleura (62), diaphragm (63), omentum (64), as well as intercostal, rhomboid and latissimus dorsi muscles (65).
Additional procedures beyond primary repair or esophagectomy may be necessary in perforation of an esophagus with underlying pathology. Relief of concomitant esophageal obstruction at the time of the repair has been shown to significantly reduce mortality (66). In particular, when there is esophageal stricture distal to the perforation, adequate dilation should be performed intraoperatively (67). In case of a non-dilatable stricture, myotomy is indicated along with fundoplication to cover the defect (68). Esophageal perforation from pneumatic dilation for achalasia requires primary repair of the perforation site and myotomy on the contralateral side of the esophagus, with a partial fundoplication procedure to prevent reflux and buttress the repair (67). In perforation of an esophagus with end-stage achalasia, the surgeon may choose to proceed with esophagectomy and reconstruction with gastric conduit if there is minimal contamination and the patient is clinically stable. In the presence of malignancy, distal obstruction requires esophageal resection with immediate or delayed reconstruction (55), while disseminated carcinoma necessitates stent placement for symptom palliation (69-71).
Esophageal T-tube placement or exclusion and diversion techniques are appropriate in clinically unstable patients and in cases where primary repair is precluded either due to preexisting esophageal disease or extensive esophageal damage. Placement of a T-tube allows establishment of a controlled esophagocutaneous fistula, thereby preventing further contamination and promoting the healing process of surrounding tissues (72). The T-tube can be removed after 4–6 weeks and the fibrous tract that was formed around the tube will eventually obliterate. Successful management of esophageal perforations with this method has been reported in many studies (3,73,74). Exclusion and diversion techniques aim to adequately drain the perforation site, minimize further contamination and expedite healing (75-77). A diversion procedure comprises of cervical esophagostomy (creation of salivary fistula), resection of the remaining esophagus, gastric decompression with gastrostomy tube, feeding tube access with a jejunostomy and closure of the diaphragmatic hiatus to prevent hernia formation (78). In critically ill patients, a cervical esophagostomy is constructed, the distal esophagus is divided at the diaphragmatic hiatus to exclude the site of perforation and a gastric feeding tube is inserted. Restoration of alimentary tract continuity is typically performed six months to one year following the perforation and usually requires retrosternal colon interposition graft (79,80). Exclusion and diversion techniques can be rather complex, highly morbid and inconvenient for the patient (3,61,68). This resulted in the development of modifications, in which the esophagus is ligated with absorbable sutures or staples, thereby obviating the need for a second operation and providing improved clinical results (81,82).
Endoscopic techniques have been recently used for the treatment of iatrogenic esophageal perforations. Endoscopic placement of covered stents aims to restore luminal integrity and prevent further extraluminal soilage (83-88). However, the effectiveness of this method depends on adequate control and drainage of the extraluminal contamination. Esophageal stenting may be appropriate in patients with extensive comorbidities, advanced mediastinal sepsis or large esophageal defects (89). Complications of this procedure include stent malposition and migration, especially when used near the gastroesophageal junction, which may cause gastric outlet obstruction. Moreover, endoscopic clipping has recently emerged as an alternative means of managing iatrogenic esophageal perforations with minimal extraluminal contamination (90). This treatment modality is generally best suited for small defects with healthy, compliant surrounding mucosa that can be approximated with minimal tension. Failure, however, to adequately control extraluminal soilage significantly increases the risk of fistula formation. Finally, topical negative-pressure therapy with an endoscopically placed vacuum sponge is a relatively new technique for the treatment of esophageal perforation. Clinical outcomes from small, retrospective studies are comparable to those of more traditional treatments; however, its safety profile is yet to be fully determined (91).
Nonoperative treatment should be reserved for patients with contained esophageal perforations, limited extraluminal soilage and no evidence of systemic inflammation. The first successful nonoperative management was described in 1965, with only 1 death in 18 patients treated for instrumental perforation of the thoracic esophagus (92). Since then, the role of nonoperative management has rapidly evolved (93-96), probably due to the increasing incidence of iatrogenic esophageal injuries, which are often associated with less extraluminal contamination. Perforation of the thoracic or abdominal esophagus can represent a relative contraindication to nonoperative management because of the difficulty in controlling spillage of intraluminal contents in the pleural or peritoneal cavities. Conversely, cervical esophageal perforation is considered suitable for nonoperative treatment due to the anatomic confinement of the esophagus by surrounding structures. Similarly, it is appropriate to consider nonoperative management if the injury is not in neoplastic tissue or proximal to an obstruction. Additional criteria include accessibility to contrast imaging studies at any time of the day and availability of an experienced surgeon if the patient deteriorates (6,93,95). Careful selection of patients with esophageal perforation for nonoperative management has achieved 100% survival rates (93,96,97).
Nonoperative treatment includes avoidance of oral intake, parenteral nutrition support, intravenous broad-spectrum antibiotics and drainage of fluid collections. If the patient remains clinically stable, a barium esophagram should be obtained after 7 days and resumption of oral intake under observation may be considered depending on the results. If the patient demonstrates any evidence of clinical deterioration, with signs and symptoms of infection, surgical intervention is required to control extraluminal contamination and restore continuity of the digestive tract. Figure 1 presents a simplified algorithm for the treatment of iatrogenic esophageal perforation.
Prognosis
The mortality of iatrogenic esophageal perforation ranges between 7% and 33% (5). Regarding the injury site, thoracic esophageal perforations have the highest mortality, followed by abdominal and cervical perforations (25,52). Nonoperative management of esophageal perforation has been associated with higher mortality compared to surgical treatment (98). The most frequently reported postoperative complications include persistent leak, mediastinitis, empyema, fistula formation, esophageal stricture, pneumonia, abscess and sepsis (23,99-102).
Conclusions
Iatrogenic esophageal perforation is a serious complication of various diagnostic and therapeutic interventions that can be challenging to diagnose and difficult to treat. As a result, its morbidity and mortality remain high. To provide improved clinical outcomes, an individualized surgical treatment is vital. In a select group of patients, however, nonoperative management can be successful. In any case, increased clinical awareness, expeditious diagnosis and optimal supportive treatment are essential.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-19-4096). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chirica M, Champault A, Dray X, et al. Esophageal perforations. J Visc Surg 2010;147:e117-28. [Crossref] [PubMed]
- Richardson JD. Management of esophageal perforations: the value of aggressive surgical treatment. Am J Surg 2005;190:161-5. [Crossref] [PubMed]
- Bufkin BL, Miller JI, Mansour KA. Esophageal perforation: emphasis on management. Ann Thorac Surg 1996;61:1447-51; discussion 1451-2. [Crossref] [PubMed]
- Barrett NR. Spontaneous perforation of the oesophagus; review of the literature and report of three new cases. Thorax 1946;1:48-70. [Crossref] [PubMed]
- Kaman L, Iqbal J, Kundil B, et al. Management of oesophageal perforation in adults. Gastroenterology Res 2010;3:235-44. [PubMed]
- Bladergroen MR, Lowe JE, Postlethwait RW. Diagnosis and recommended management of esophageal perforation and rupture. Ann Thorac Surg 1986;42:235-9. [Crossref] [PubMed]
- Sdralis EIK, Petousis S, Rashid F, et al. Epidemiology, diagnosis, and management of esophageal perforations: systematic review. Dis esophagus Off J Int Soc Dis Esophagus 2017;30:1-6. [Crossref] [PubMed]
- Kavic SM, Basson MD. Complications of endoscopy. Am J Surg 2001;181:319-32. [Crossref] [PubMed]
- Silvis SE, Nebel O, Rogers G, et al. Endoscopic complications. Results of the 1974 American Society for Gastrointestinal Endoscopy Survey. JAMA 1976;235:928-30. [Crossref] [PubMed]
- Vermeulen BD, Siersema PD. Esophageal Stenting in Clinical Practice: an Overview. Curr Treat Options Gastroenterol 2018;16:260-73. [Crossref] [PubMed]
- ASGE Standards of Practice Committee, Ben-Menachem T, Decker GA, et al. Adverse events of upper GI endoscopy. Gastrointest Endosc 2012;76:707-18. [Crossref] [PubMed]
- Manner H, May A, Miehlke S, et al. Ablation of nonneoplastic Barrett’s mucosa using argon plasma coagulation with concomitant esomeprazole therapy (APBANEX): a prospective multicenter evaluation. Am J Gastroenterol 2006;101:1762-9. [Crossref] [PubMed]
- Litle VR, Luketich JD, Christie NA, et al. Photodynamic therapy as palliation for esophageal cancer: experience in 215 patients. Ann Thorac Surg 2003;76:1687-92; discussion 1692-3.
- Boeckxstaens GE, Annese V, Des Varannes SB, et al. Pneumatic dilation versus laparoscopic heller’s myotomy for idiopathic achalasia. N Engl J Med 2011;364:1807-16. [Crossref] [PubMed]
- Moss A, Bourke MJ, Hourigan LF, et al. Endoscopic resection for Barrett’s high-grade dysplasia and early esophageal adenocarcinoma: an essential staging procedure with long-term therapeutic benefit. Am J Gastroenterol 2010;105:1276-83. [Crossref] [PubMed]
- Pouw RE, Seewald S, Gondrie JJ, et al. Stepwise radical endoscopic resection for eradication of Barrett’s oesophagus with early neoplasia in a cohort of 169 patients. Gut 2010;59:1169-77. [Crossref] [PubMed]
- Campos GM, Vittinghoff E, Rabl C, et al. Endoscopic and surgical treatments for achalasia: a systematic review and meta-analysis. Ann Surg 2009;249:45-57. [Crossref] [PubMed]
- Schmitz RJ, Sharma P, Badr AS, et al. Incidence and management of esophageal stricture formation, ulcer bleeding, perforation, and massive hematoma formation from sclerotherapy versus band ligation. Am J Gastroenterol 2001;96:437-41. [Crossref] [PubMed]
- Lightdale CJ, Heier SK, Marcon NE, et al. Photodynamic therapy with porfimer sodium versus thermal ablation therapy with Nd:YAG laser for palliation of esophageal cancer: a multicenter randomized trial. Gastrointest Endosc 1995;42:507-12. [Crossref] [PubMed]
- Korula J, Pandya K, Yamada S. Perforation of esophagus after endoscopic variceal sclerotherapy. Incidence and clues to pathogenesis. Dig Dis Sci 1989;34:324-9. [Crossref] [PubMed]
- Copenhagen Esophageal Varices Sclerotherapy Project. Sclerotherapy after first variceal hemorrhage in cirrhosis. A randomized multicenter trial. N Engl J Med 1984;311:1594-600. [Crossref] [PubMed]
- Michel L, Grillo HC, Malt RA. Operative and nonoperative management of esophageal perforations. Ann Surg 1981;194:57-63. [Crossref] [PubMed]
- Skinner DB, Little AG, DeMeester TR. Management of esophageal perforation. Am J Surg 1980;139:760-4. [Crossref] [PubMed]
- Gupta NM, Kaman L. Personal management of 57 consecutive patients with esophageal perforation. Am J Surg 2004;187:58-63. [Crossref] [PubMed]
- Jones WG, Ginsberg RJ. Esophageal perforation: a continuing challenge. Ann Thorac Surg 1992;53:534-43. [Crossref] [PubMed]
- Doll N, Borger MA, Fabricius A, et al. Esophageal perforation during left atrial radiofrequency ablation: Is the risk too high? J Thorac Cardiovasc Surg 2003;125:836-42. [Crossref] [PubMed]
- Gaudinez RF, English GM, Gebhard JS, et al. Esophageal perforations after anterior cervical surgery. J Spinal Disord 2000;13:77-84. [Crossref] [PubMed]
- Venuta F, Rendina EA, De Giacomo T, et al. Esophageal perforation after sequential double-lung transplantation. Chest 2000;117:285-7. [Crossref] [PubMed]
- McBurney RP. Perforation of the esophagus: a complication of vagotomy or hiatal hernia repair. Ann Surg 1969;169:851-6. [Crossref] [PubMed]
- Postlethwait RW, Kim SK, Dillon ML. Esophageal complications of vagotomy. Surg Gynecol Obstet 1969;128:481-8. [PubMed]
- Vrouenraets BC, Been HD, Brouwer-Mladin R, et al. Esophageal perforation associated with cervical spine surgery: report of two cases and review of the literature. Dig Surg 2004;21:246-9. [Crossref] [PubMed]
- Ruetzler K, Krafft P, Frass M. Airway Management in Intensive Care Medicine. In: Hagberg CA. editor. Benumof and Hagberg’s Airway Management. 3rd ed. W.B. Saunders; 2013:916-954.e7.
- Lee JG, Lieberman DA. Complications related to endoscopic hemostasis techniques. Gastrointest Endosc Clin N Am 1996;6:305-21. [Crossref] [PubMed]
- Jougon J, Cantini O, Delcambre F, et al. Esophageal perforation: life threatening complication of endotracheal intubation. Eur J Cardiothorac Surg 2001;20:7-10; discussion 10-1. [Crossref] [PubMed]
- Kallmeyer IJ, Collard CD, Fox JA, et al. The safety of intraoperative transesophageal echocardiography: a case series of 7200 cardiac surgical patients. Anesth Analg 2001;92:1126-30. [Crossref] [PubMed]
- Ahmed A, Aggarwal M, Watson E. Esophageal perforation: a complication of nasogastric tube placement. Am J Emerg Med 1998;16:64-6. [Crossref] [PubMed]
- Allen PW, Thornton M. Oesophageal perforation with minitracheostomy. Intensive Care Med 1989;15:543. [Crossref] [PubMed]
- Sarr MG, Pemberton JH, Payne WS. Management of instrumental perforations of the esophagus. J Thorac Cardiovasc Surg 1982;84:211-8. [Crossref] [PubMed]
- Attar S, Hankins JR, Suter CM, et al. Esophageal perforation: a therapeutic challenge. Ann Thorac Surg 1990;50:45-9; discussion 50-1. [Crossref] [PubMed]
- Goldstein LA, Thompson WR. Esophageal perforations: a 15 year experience. Am J Surg 1982;143:495-503. [Crossref] [PubMed]
- Brewer LA, Carter R, Mulder GA, et al. Options in the management of perforations of the esophagus. Am J Surg 1986;152:62-9. [Crossref] [PubMed]
- Altorjay A, Kiss J, Vörös A, et al. The role of esophagectomy in the management of esophageal perforations. Ann Thorac Surg 1998;65:1433-6. [Crossref] [PubMed]
- Wang N, Razzouk AJ, Safavi A, et al. Delayed primary repair of intrathoracic esophageal perforation: is it safe? J Thorac Cardiovasc Surg 1996;111:114-21; discussion 121-2. [Crossref] [PubMed]
- Rubesin SE, Levine MS. Radiologic diagnosis of gastrointestinal perforation. Radiol Clin North Am 2003;41:1095-115. v. [Crossref] [PubMed]
- Okten I, Cangir AK, Ozdemir N, et al. Management of esophageal perforation. Surg Today 2001;31:36-9. [Crossref] [PubMed]
- Panzini L, Burrell MI, Traube M. Instrumental esophageal perforation: chest film findings. Am J Gastroenterol 1994;89:367-70. [PubMed]
- Foley MJ, Ghahremani GG, Rogers LF. Reappraisal of contrast media used to detect upper gastrointestinal perforations: comparison of ionic water-soluble media with barium sulfate. Radiology 1982;144:231-7. [Crossref] [PubMed]
- Maniatis V, Chryssikopoulos H, Roussakis A, et al. Perforation of the alimentary tract: evaluation with computed tomography. Abdom Imaging 2000;25:373-9. [Crossref] [PubMed]
- Backer CL, LoCicero J, Hartz RS, et al. Computed tomography in patients with esophageal perforation. Chest 1990;98:1078-80. [Crossref] [PubMed]
- Maher MM, Lucey BC, Boland G, et al. The role of interventional radiology in the treatment of mediastinal collections caused by esophageal anastomotic leaks. AJR Am J Roentgenol 2002;178:649-53. [Crossref] [PubMed]
- Pasricha PJ, Fleischer DE, Kalloo AN. Endoscopic perforations of the upper digestive tract: a review of their pathogenesis, prevention, and management. Gastroenterology 1994;106:787-802. [Crossref] [PubMed]
- Brinster CJ, Singhal S, Lee L, et al. Evolving options in the management of esophageal perforation. Ann Thorac Surg 2004;77:1475-83. [Crossref] [PubMed]
- Gupta NM. Emergency transhiatal oesophagectomy for instrumental perforation of an obstructed thoracic oesophagus. Br J Surg 1996;83:1007-9. [Crossref] [PubMed]
- White RK, Morris DM. Diagnosis and management of esophageal perforations. Am Surg 1992;58:112-9. [PubMed]
- Orringer MB, Stirling MC. Esophagectomy for esophageal disruption. Ann Thorac Surg 1990;49:35-42; discussion 42-3. [Crossref] [PubMed]
- Ikeda Y, Niimi M, Sasaki Y, et al. Thoracoscopic repair of a spontaneous perforation of the esophagus with the endoscopic suturing device. J Thorac Cardiovasc Surg 2001;121:178-9. [Crossref] [PubMed]
- Kiel T, Ferzli G, McGinn J. The use of thoracoscopy in the treatment of iatrogenic esophageal perforations. Chest 1993;103:1905-6. [Crossref] [PubMed]
- Whyte RI, Iannettoni MD, Orringer MB. Intrathoracic esophageal perforation. The merit of primary repair. J Thorac Cardiovasc Surg 1995;109:140-4; discussion 144-6. [Crossref] [PubMed]
- Ohri SK, Liakakos TA, Pathi V, et al. Primary repair of iatrogenic thoracic esophageal perforation and Boerhaave’s syndrome. Ann Thorac Surg 1993;55:603-6. [Crossref] [PubMed]
- Nesbitt JC, Sawyers JL. Surgical management of esophageal perforation. Am Surg 1987;53:183-91. [PubMed]
- Gouge TH, Depan HJ, Spencer FC. Experience with the Grillo pleural wrap procedure in 18 patients with perforation of the thoracic esophagus. Ann Surg 1989;209:612-7; discussion 617-9. [Crossref] [PubMed]
- Grillo HC, Wilkins EW. Esophageal repair following late diagnosis of intrathoracic perforation. Ann Thorac Surg 1975;20:387-99. [Crossref] [PubMed]
- Kotsis L, Agócs L. The effectiveness of diaphragmatic pedicled grafts in esophageal injuries and wall reconstruction. Eur J Cardiothorac Surg 1998;14:218-20. [PubMed]
- Sabanathan S, Eng J, Richardson J. Surgical management of intrathoracic oesophageal rupture. Br J Surg 1994;81:863-5. [Crossref] [PubMed]
- Richardson JD, Tobin GR. Closure of esophageal defects with muscle flaps. Arch Surg 1994;129:541-7; discussion 547-8. [Crossref] [PubMed]
- Moghissi K, Pender D. Instrumental perforations of the oesophagus and their management. Thorax 1988;43:642-6. [Crossref] [PubMed]
- Urbani M, Mathisen DJ. Repair of esophageal perforation after treatment for achalasia. Ann Thorac Surg 2000;69:1609-11. [Crossref] [PubMed]
- Søreide JA, Viste A. Esophageal perforation: diagnostic work-up and clinical decision-making in the first 24 hours. Scand J Trauma Resusc Emerg Med 2011;19:66. [Crossref] [PubMed]
- Liedman B, Johnsson E, Lundell L. Treatment of iatrogenic perforations with covered stents in patients with oesophageal cancer. Eur J Surg 2001;167:672-4. [Crossref] [PubMed]
- Morgan RA, Ellul JP, Denton ER, et al. Malignant esophageal fistulas and perforations: management with plastic-covered metallic endoprostheses. Radiology 1997;204:527-32. [Crossref] [PubMed]
- Feins RH, Johnstone DW, Baronos ES, et al. Palliation of inoperable esophageal carcinoma with the Wallstent endoprosthesis. Ann Thorac Surg 1996;62:1603-7. [Crossref] [PubMed]
- Qadir I, Zafar H, Khan MZ, et al. T-tube management of late esophageal perforation. J Pak Med Assoc 2011;61:418-20. [PubMed]
- Ojima H, Kuwano H, Sasaki S, et al. Successful late management of spontaneous esophageal rupture using T-tube mediastinoabdominal drainage. Am J Surg 2001;182:192-6. [Crossref] [PubMed]
- Naylor AR, Walker WS, Dark J, et al. T tube intubation in the management of seriously ill patients with oesophagopleural fistulae. Br J Surg 1990;77:40-2. [Crossref] [PubMed]
- Kiernan PD, Rhee J, Collazo L, et al. Complete esophageal diversion: a simplified, easily reversible technique. J Am Coll Surg 2005;200:812-author reply 812-3. [Crossref] [PubMed]
- Urschel HC, Razzuk MA, Wood RE, et al. Improved management of esophageal perforation: exclusion and diversion in continuity. Ann Surg 1974;179:587-91. [Crossref] [PubMed]
- Menguy R. Near-total esophageal exclusion by cervical esophagostomy and tube gastrostomy in the management of massive esophageal perforation: report of a case. Ann Surg 1971;173:613-6. [Crossref] [PubMed]
- Rohatgi A, Papanikitas J, Sutcliffe R, et al. The role of oesophageal diversion and exclusion in the management of oesophageal perforations. Int J Surg 2009;7:142-4. [Crossref] [PubMed]
- Fürst H, Hartl WH, Löhe F, et al. Colon interposition for esophageal replacement: an alternative technique based on the use of the right colon. Ann Surg 2000;231:173-8. [Crossref] [PubMed]
- Thomas P, Fuentes P, Giudicelli R, et al. Colon interposition for esophageal replacement: current indications and long-term function. Ann Thorac Surg 1997;64:757-64. [Crossref] [PubMed]
- Bardini R, Bonavina L, Pavanello M, et al. Temporary double exclusion of the perforated esophagus using absorbable staples. Ann Thorac Surg 1992;54:1165-7. [Crossref] [PubMed]
- Lee YC, Lee ST, Chu SH. New technique of esophageal exclusion for chronic esophageal perforation. Ann Thorac Surg 1991;51:1020-2. [Crossref] [PubMed]
- Schmidt SC, Strauch S, Rösch T, et al. Management of esophageal perforations. Surg Endosc 2010;24:2809-13. [Crossref] [PubMed]
- Salminen P, Gullichsen R, Laine S. Use of self-expandable metal stents for the treatment of esophageal perforations and anastomotic leaks. Surg Endosc 2009;23:1526-30. [Crossref] [PubMed]
- Tuebergen D, Rijcken E, Mennigen R, et al. Treatment of thoracic esophageal anastomotic leaks and esophageal perforations with endoluminal stents: efficacy and current limitations. J Gastrointest Surg 2008;12:1168-76. [Crossref] [PubMed]
- Freeman RK, Van Woerkom JM, Ascioti AJ. Esophageal stent placement for the treatment of iatrogenic intrathoracic esophageal perforation. Ann Thorac Surg 2007;83:2003-7; discussion 2007-8.
- Fischer A, Thomusch O, Benz S, et al. Nonoperative treatment of 15 benign esophageal perforations with self-expandable covered metal stents. Ann Thorac Surg 2006;81:467-72. [Crossref] [PubMed]
- White RE, Mungatana C, Topazian M. Expandable stents for iatrogenic perforation of esophageal malignancies. J Gastrointest Surg 2003;7:715-9; discussion 719-20. [Crossref] [PubMed]
- Sharma P, Kozarek R. Practice Parameters Committee of American College of Gastroenterology. Role of esophageal stents in benign and malignant diseases. Am J Gastroenterol 2010;105:258-73. [Crossref] [PubMed]
- Qadeer MA, Dumot JA, Vargo JJ, et al. Endoscopic clips for closing esophageal perforations: case report and pooled analysis. Gastrointest Endosc 2007;66:605-11. [Crossref] [PubMed]
- Newton NJ, Sharrock A, Rickard R, et al. Systematic review of the use of endo-luminal topical negative pressure in oesophageal leaks and perforations. Dis esophagus Off J Int Soc Dis Esophagus 2017;30:1-5. [PubMed]
- Mengold L, Klassen K. Conservative management of esophageal perforation. Arch Surg 1965;91:232-40.
- Altorjay A, Kiss J, Vörös A, et al. Nonoperative management of esophageal perforations. Is it justified? Ann Surg 1997;225:415-21. [Crossref] [PubMed]
- Ballesta-Lopez C, Vallet-Fernandez J, Catarci M, et al. Iatrogenic perforations of the esophagus. Int Surg 1993;78:28-31. [PubMed]
- Shaffer HA, Valenzuela G, Mittal RK. Esophageal perforation. A reassessment of the criteria for choosing medical or surgical therapy. Arch Intern Med 1992;152:757-61. [Crossref] [PubMed]
- Cameron JL, Kieffer RF, Hendrix TR, et al. Selective nonoperative management of contained intrathoracic esophageal disruptions. Ann Thorac Surg 1979;27:404-8. [Crossref] [PubMed]
- Vogel SB, Rout WR, Martin TD, et al. Esophageal perforation in adults: aggressive, conservative treatment lowers morbidity and mortality. Ann Surg 2005;241:1016-21; discussion 1021-3. [Crossref] [PubMed]
- Eroglu A, Turkyilmaz A, Aydin Y, et al. Current management of esophageal perforation: 20 years experience. Dis esophagus Off J Int Soc Dis Esophagus 2009;22:374-80. [Crossref] [PubMed]
- Port JL, Kent MS, Korst RJ, et al. Thoracic esophageal perforations: a decade of experience. Ann Thorac Surg 2003;75:1071-4. [Crossref] [PubMed]
- Safavi A, Wang N, Razzouk A, et al. One-stage primary repair of distal esophageal perforation using fundic wrap. Am Surg 1995;61:919-24. [PubMed]
- Salo JA, Isolauri JO, Heikkilä LJ, et al. Management of delayed esophageal perforation with mediastinal sepsis. Esophagectomy or primary repair? J Thorac Cardiovasc Surg 1993;106:1088-91. [Crossref] [PubMed]
- Kim-Deobald J, Kozarek RA. Esophageal perforation: an 8-year review of a multispecialty clinic’s experience. Am J Gastroenterol 1992;87:1112-9. [PubMed]


