
Pulmonary metastasectomy in the era of targeted therapy and immunotherapy
Introduction
In 2018, there were 287,723 cases of new cutaneous melanoma diagnosed worldwide and 60,712 deaths occurred attributable to the disease. Australia has the highest incidence of cutaneous melanoma in the world—33.6 per 100,000 person-years (1). Risk factors for melanoma include sunburns due to ultraviolet (UV) light exposure, the presence of melanocytic or dysplastic naevi, a personal and/or family history of cutaneous melanoma and fair skin-type (2). It is estimated that from data available from the United States, 4% of all melanoma diagnosed present with stage IV disease with distant metastases (3). The estimated 5-year survival of metastatic melanoma in 2015 was 6%, and the estimated median overall survival was 7.5 months (4). The most common site of metastases is the lungs, affecting up to 30% of patients with metastatic disease (5).
Previously, metastatic melanoma heralded a grim prognosis due to the ineffectiveness of chemotherapy (6). Surgical treatment of metastases rarely occurred as distant metastases precluded a curative resection and if performed, it was typically reserved for a palliative intent (7). However, in selected patients with limited volume metastases, surgery was performed with a curative intent if a complete resection of metastases could be achieved. In selected patients with pulmonary metastases, a median survival of up to 18.3 months, and 5-year survival rates of up to 35.1% were achieved (8). The failure to identify effective chemotherapy agents coupled with the growing data on the immunogenicity of melanoma led to interest in immunotherapy, focused mostly around immunomodulatory cytokines such as interferon and interleukin-2. However, these agents failed to result in any significant tumour response nor improve survival (9). Recently, the introduction of new targeted and immunotherapies including BRAF- and MEK-inhibitors in association with programmed cell death (PD-1) inhibitors and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) has substantially improved outcomes of patients with metastatic disease (10).
With the significant survival gains observed, the role of surgery in patients with stage IV disease needs to be re-examined. A recent study from the Royal Marsden Hospital, in London, assessed patients undergoing surgery for stage IV melanoma before the time of effective systemic therapies (2003–2007) compared to after (2011–2015) with 69 patients in each group. The authors report the indication for surgery showed trends towards an increase in abdominal metastasectomy, decreased in-transit lesion excision and an increase in potentially curative operations for residual oligometastatic disease (11), suggesting a paradigm shift in the role of surgery aiming for cure of metastatic melanoma. The improved tumour responses to the latest systemic therapies have achieved more effective systemic disease control that now allows surgical metastasectomy, in patients where surgery would ever have been performed. Therefore, the introduction of effective systemic therapies in metastatic melanoma requires a re-evaluation of the role of surgery. In this review, we aim to describe the progress in oncologic management of metastatic melanoma, review the role of pulmonary metastasectomy for metastatic melanoma and clarify the role of pulmonary metastasectomy in the current era of effective systemic therapy to provide best practice based on current evidence.
Medical management
Medical management can be divided into three broad categories: chemotherapy, immunotherapy and targeted therapy. A comparison of those therapies (and their combinations) are summarised in Figure 1. Metastatic melanoma had a grim prognosis before the advent of effective targeted immunotherapies. The first chemotherapy trials for metastatic melanoma began in the 1960s, using 1-phenylalanine mustard, also known as melphalan (41). Melphalan, however, proved to be highly toxic and ineffective. The Hunterian Lecture, of the Royal College of Surgeons of England, delivered in 1968, refers to 29 cases of metastatic melanoma that were treated with melphalan and noted several limitations, namely the dependence on anatomical arterial supply, which may not correspond with the metastatic deposit, the high general toxicity and the rapid recurrence rate after treatment (42). From 1975 onwards, dacarbazine was the chemotherapeutic agent of choice. It has been extensively investigated and remains the only FDA-approved chemotherapeutic agent for this purpose (43). Despite its ubiquity in a variety of regimens in the pre-immunotherapy era, disease response was poor and temporary in most patients. Hill et al. found that less than 2% of patients treated with dacarbazine alone were alive at 6 years (44). Combination treatments involving dacarbazine, including the Dartmouth regimen (consisting of cisplatin, dacarbazine, carmustine and tamoxifen) were extensively studied, but did not yield a survival benefit over dacarbazine monotherapy (12), despite having an improved overall response rates of 26% versus 5% for dacarbazine monotherapy (45). Temozolomide, an orally-administered dacarbazine analogue, garnered attention due to superior bioavailability and central nervous system (CNS) penetration compared to dacarbazine (46). The tumour response and survival rates of patients with CNS metastases treated by temozolomide however, resembled that of patients receiving dacarbazine (47). Chiarion-Sileni et al. showed that the rates of brain metastases were unchanged when treated with temozolomide or dacarbazine in combination with cisplatin and interleukin-2 (IL-2) (48).
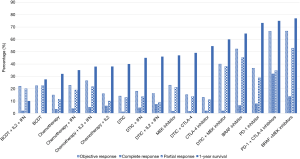
Immunotherapy for metastatic melanoma initially focused on immunomodulation, via administration of cytokines. Interferon (IFN), an endogenous protein that stimulates the immune system to recognise foreign proteins, was shown to be able to recognise and destroy melanoma cells. However, it did not improve response or survival in patients with metastatic disease. A phase III randomized study of 4 weeks of intravenous interferon-a-2b versus observation conducted by the Eastern Cooperative Oncology Group showed no discernible difference between the two treatment groups in terms of relapse-free or overall survival (P values of 0.964 and 0.558, and hazard ratios for interferon versus observation of 0.98 and 1.08 respectively) (49). IL-2 is a cytokine that promotes the proliferation of melanoma-specific T-cells. It was first approved for use in metastatic disease in 1992. It has been shown that approximately 6% of patients can achieve a complete response to treatment. Although this represented only a small subset of patients, 60% of those complete responders had durable responses lasting beyond 122 months (50). IL-2 treatment is however highly toxic, and strategies to identify patients who are more likely to respond have proven elusive (51). Attempts to combine interferon and interleukin with chemotherapy have also been unsuccessful. A trial coordinated by the Eastern Cooperative Oncology Group failed to find an improvement in overall survival or durable responses after comparing chemotherapy combined with interferon and interleukin with chemotherapy alone (median overall survival 8.7 vs. 9 months respectively) (13).
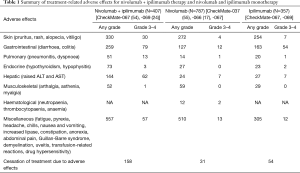
The current renaissance in treatments of stage IV melanoma occurred following the introduction of checkpoint blockade, particularly to CTLA-4 and PD-1; and novel targeted therapies to BRAF and MEK. Melanoma cells are highly mutagenic, and quickly evolve mechanisms to evade the immune system. The principles of checkpoint blockade therefore involve antibodies to those molecular pathways that help melanoma escape immunity. Some melanoma variants overexpress programmed cell death protein 1 ligand (PD-L1/2), which binds to PD-1 on T-cells (9). This allows such variants to masquerade as self-cells and evade the immune response. Nivolumab and pembrolizumab are the two anti-PD-1 drugs that have been approved for use in metastatic melanoma (41). CTLA-4 is expressed on activated T-cells and regulatory T-cells, acting to suppress T-cell activity when bound to B7-1/2 (52). Ipilimumab is the only anti-CTLA-4 drug approved for use in metastatic disease. Overall, PD-1 inhibitors are superior to ipilimumab in efficacy and side-effect tolerance (2). However, ipilimumab combined with nivolumab is superior to ipilimumab and nivolumab monotherapy (53). Results from the phase 3 Checkmate 067 trial showed an unprecedented 60-month median survival with combination therapy compared to monotherapy alone (14). However, combination therapy is associated with a severe side-effect profile, necessitating more stringent monitoring. The side effects can range from mild skin manifestations like pruritus and dermatitis, to life-threatening complications like colitis, neutropaenia, agranulocytosis and cardiac dysfunction. A summary of these side effects is listed in Table 1. While most side effects are managed safely with glucocorticoid therapy, severe manifestations require immunosuppression (56). Reassuringly, the Checkmate 067 trial found no association with early cessation of combined therapy for adverse events and diminished overall and progression-free survival. Together, this suggests that the efficacy of immunotherapy may be long-lasting. Preliminary findings of the concurrent Checkmate 511 trial comparing nivolumab 3 mg/kg plus ipilimumab 1 mg/kg (NIVO3 + IPI1) to the first-line treatment of Nivolumab 1 mg/kg plus ipilimumab 3 mg/kg (NIVO1 + IPI3) have yielded decreased side effect profiles when treated with low-dose ipilimumab. In particular, the authors describe lower rates of diarrhoea, colitis and transaminitis. The duration of treatment was also longer, consistent with a lower rate of discontinuation. Long-term surveillance to assess survival of patients on the NIVO3+IPI1 regimen are ongoing, but preliminary results have shown similar overall and progression-free survival between the two groups.
Full table
Novel targeted therapies to BRAFv600 and MEK represent the cutting edge in treatments for BRAF-mutated melanoma. Treatment with BRAF and MEK inhibitors show rapid, durable responses regardless of tumour burden and location of metastases. Pooled data from the COMBI-d and COMBI-v trials demonstrated that 19% of patients treated with Dabrafenib and Trametinib combination therapy were still alive at 5-year follow-up. A complete response occurred in another 19% of patients, and this was associated with a survival rate of 71% at 5 years (15). However, patients who have BRAF wild-type melanoma have poor responses to targeted therapy, and treatment options for this population are scarce should immunotherapies also fail (16). Targeted therapy also induces resistance through several pathways, which include BRAF gene amplifications and MEK1 and MEK2 mutations (57). Menzies et al. showed that 75% of patients treated with combination BRAF and MEK inhibition progress to new metastases, even with complete responses to their initial disease burden (58), signalling an urgent need for development of new therapies for this patient population. At present, resistant clones represent a significant challenge to effective targeted therapies. Whilst theoretical inhibitory targets exist within the MAPK pathway and to its downstream targets like ERK and CDK4 (57), no current effective therapies exist for resistant clones. However, the effectiveness of combined BRAF and MEK inhibition mean that they are now first-line treatment in patients with BRAFv600 mutated melanoma. The two treatments currently available are vemurafenib and cobimetinib, and dabrafenib and trametinib. Patients with wild-type BRAF melanomas have limited options—for such patients anti-PD1 immunotherapy is the initial agent of choice (17).
Surgical management
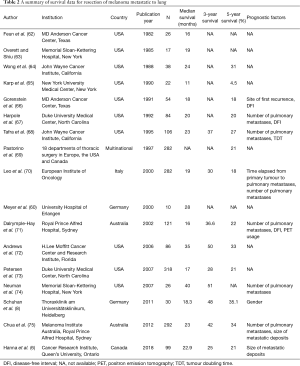
Curative surgery for metastatic melanoma achieved by complete resection of metastasis, where possible, as previously stated has been associated with improved outcomes over traditional chemotherapy. A cohort analysis of 1,574 patients over 29 years at the John Wayne Cancer Institute demonstrated favourable survival rates for patients with distant skin, subcutaneous or lymph node metastases (median survival 35.1 months), gastrointestinal tract (GIT) metastases (median survival 36.7 months), and lung metastases (median survival 28.1 months). Patients with adrenal, brain and liver metastases had lower median survival rates (27.4, 21.9 and 18.2 months respectively) (59). A comparison between combination chemo- and immunotherapy versus curative surgery reported by Meyer et al. showed an improved median survival rate of 17 months in patients receiving curative resections regardless of metastatic sites, versus 5 months for patients receiving non-surgical treatments (60). The survival benefits conferred by curative resections are seen even in comparison to patients receiving CTLA-4 and PD-1 inhibitors. Deutsch et al. showed that patients who underwent metastasectomies of intestinal metastases achieved a median overall survival (OS) of 64 months, compared to 11 months when patients are treated by ipilimumab, nivolumab and pembrolizumab (61). A summary of the effectiveness of resection of pulmonary metastasis (AJCC 8th edition Stage IV M1b melanoma) are summarised in Table 2.
Full table
Patient selection for surgery represents a major hurdle in the provision of treatment. The number of distant metastases greatly influence survival rate. Surgery is therefore best suited for patients who have limited metastatic deposits at a single organ. The volume of the disease is important as reported by Tafra et al. who demonstrated that patients with solitary disease were more likely to be alive at 5-year follow-up compared to patients with multiple metastatic deposits following pulmonary metastasectomy (68). Also patients who are significantly comorbid or who have shown limited responses to chemo-immunotherapy are less likely to benefit from surgery (76). In addition, the biology as demonstrated by tumour doubling time (TDT) is an important factor regarding patient selection for pulmonary metastasectomy. The relationship between TDTs and survival is likely defined by the body’s immune response to the disease and is a measure of immunocompetence—if the TDT is rapid, it can be inferred that this implies a poor immune response and outcomes are therefore poor (70). Patients with TDTs of less than 60 days had a 5-year survival of 0 percent, whereas TDTs equal to or exceeding 60 days had a 5-year survival of 20.7% (77). The stringent selection criteria for surgery leads to a selection bias, as patients who fulfil the criteria are likely to be highly selected and with low volume metastases (78), which ultimately implies a more favourable disease profile that will most likely translate to better outcomes.
In the era of effective immunotherapies and targeted therapies further re-examination of the role of surgery is required. Effective targeted therapies have provided unprecedented survival rates. This effectiveness is seen also in patients with low volume disease, a demographic that was traditionally most effectively treated with surgery (79). A pilot study of 19 patients by He et al. showed durable responses in patients who were treated with metastasectomies combined with treatment by the BRAF inhibitor vemurafenib for more than 12 months (80). While the study was limited by a small sample size, it nevertheless portends an evolution in the role of surgery to complement targeted therapies, rather than being rendered obsolete by it. Recent reports have demonstrated an increasing role of surgery in the treatment of clones resistant to BRAF and MEK inhibitors (11), with one study reporting three of 69 patients (4.3%) requiring metastectomies to remove clones resistant to immunotherapy and/or checkpoint inhibition. This mirrors the historical role of surgery as a last resort in most kinds of pathology when all other options are either exhausted or non-existent.
Systemic therapy now affords survival rates rivalling and even exceeding that of surgery in those selected cases in the past and even for patients with widespread metastases. The treatment strategies for lung metastases today has become complex. Without any available consensus guidelines, pulmonary metastasectomy must be discussed within the setting of a multi-disciplinary team comprising of surgeons, medical oncologists, radiologists, pathologists and other clinicians. If the melanoma lung metastasis is localised, the possible treatment options would include resection and adjuvant therapy or neoadjuvant therapy and pulmonary metastasectomy if the disease persists but without progression. In selected patients with widespread disease with partial tumour response to systemic therapy, consideration for surgery may be made if the disease is low volume, is amendable to complete metastasectomy and the surgery carries acceptable morbidity profile.
In conclusion, surgery remains relevant in the era of effective immunotherapies and targeted therapies for metastatic melanoma, despite their unprecedented effectiveness. Modern medical therapies are associated with a side effect profile that may limit wider application. Surgical resection of pulmonary metastases will remain relevant specifically to achieve complete resection of metastases in patients with low volume metastatic disease and to address resistant clones after targeted therapy and immunotherapy. Together, the combined adoption of multimodality therapy with surgery, targeted therapies and immunotherapy has the potential to maximise melanoma survival outcomes.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Khosro Hekmat) for the series “Pulmonary Metastases” published in Journal of Thoracic Disease. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.120). The series “Pulmonary Metastases” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- International Agency for Research on Cancer WHOW, Global Cancer Observatory Estimated age-standardized incidence rates (World) in 2018, melanoma of skin, both sexes, all ages. Available online: http://gco.iarc.fr/. Accessed 25/08/2019
- Schadendorf D, van Akkooi ACJ, Berking C, et al. Melanoma. Lancet 2018;392:971-84. [Crossref] [PubMed]
- Wei IH, Healy MA, Wong SL. Surgical treatment options for stage IV melanoma. Surg Clin North Am 2014;94:1075-89. ix. [Crossref] [PubMed]
- Deutsch GB, Kirchoff DD, Faries MB. Metastasectomy for stage IV melanoma. Surg Oncol Clin N Am 2015;24:279-98. [Crossref] [PubMed]
- Viehof J, Livingstone E, Loscha E, et al. Prognostic factors for pulmonary metastasectomy in malignant melanoma: size matters. Eur J Cardiothorac Surg 2019;56:1104-9. [Crossref] [PubMed]
- Hanna TP, Chauvin C, Miao Q, et al. Clinical Outcomes After Pulmonary Metastasectomy for Melanoma: A Population-Based Study. Ann Thorac Surg 2018;106:1675-81. [Crossref] [PubMed]
- van Akkooi AC, Atkins MB, Agarwala SS, et al. Surgical Management and Adjuvant Therapy for High-Risk and Metastatic Melanoma. Am Soc Clin Oncol Educ Book 2016;35:e505-14. [Crossref] [PubMed]
- Schuhan C, Muley T, Dienemann H, et al. Survival after pulmonary metastasectomy in patients with malignant melanoma. Thorac Cardiovasc Surg 2011;59:158-62. [Crossref] [PubMed]
- Passarelli A, Mannavola F, Stucci LS, et al. Immune system and melanoma biology: a balance between immunosurveillance and immune escape. Oncotarget 2017;8:106132-42. [Crossref] [PubMed]
- Silva IP, Long GV. Systemic therapy in advanced melanoma: integrating targeted therapy and immunotherapy into clinical practice. Curr Opin Oncol 2017;29:484-92. [Crossref] [PubMed]
- Smith MJF, Smith HG, Joshi K, et al. The impact of effective systemic therapies on surgery for stage IV melanoma. Eur J Cancer 2018;103:24-31. [Crossref] [PubMed]
- Chapman PB, Einhorn LH, Meyers ML, et al. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol 1999;17:2745-51. [Crossref] [PubMed]
- Atkins MB, Hsu J, Lee S, et al. Phase III trial comparing concurrent biochemotherapy with cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon alfa-2b with cisplatin, vinblastine, and dacarbazine alone in patients with metastatic malignant melanoma (E3695): a trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol 2008;26:5748-54. [Crossref] [PubMed]
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med 2019;381:1535-46. [Crossref] [PubMed]
- Robert C, Grob JJ, Stroyakovskiy D, et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N Engl J Med 2019;381:626-36. [Crossref] [PubMed]
- Dummer R, Schadendorf D, Ascierto PA, et al. Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2017;18:435-45. [Crossref] [PubMed]
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320-30. [Crossref] [PubMed]
- Young AM, Marsden J, Goodman A, et al. Prospective randomized comparison of dacarbazine (DTIC) versus DTIC plus interferon-alpha (IFN-alpha) in metastatic melanoma. Clin Oncol (R Coll Radiol) 2001;13:458-65. [PubMed]
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015;372:30-9. [Crossref] [PubMed]
- Robert C, Ribas A, Schachter J, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol 2019;20:1239-51. [Crossref] [PubMed]
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517-26. [Crossref] [PubMed]
- Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015;16:908-18. [Crossref] [PubMed]
- Ribas A, Kefford R, Marshall MA, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol 2013;31:616-22. [Crossref] [PubMed]
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006-17. [Crossref] [PubMed]
- McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol 2014;15:323-32. [Crossref] [PubMed]
- Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet 2015;386:444-51. [Crossref] [PubMed]
- Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 2014;371:1867-76. [Crossref] [PubMed]
- Keilholz U, Punt CJ, Gore M, et al. Dacarbazine, cisplatin, and interferon-alfa-2b with or without interleukin-2 in metastatic melanoma: a randomized phase III trial (18951) of the European Organisation for Research and Treatment of Cancer Melanoma Group. J Clin Oncol 2005;23:6747-55. [Crossref] [PubMed]
- Johnston SR, Constenla DO, Moore J, et al. Randomized phase II trial of BCDT [carmustine (BCNU), cisplatin, dacarbazine (DTIC) and tamoxifen] with or without interferon alpha (IFN-alpha) and interleukin (IL-2) in patients with metastatic melanoma. Br J Cancer 1998;77:1280-6. [Crossref] [PubMed]
- Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 2016;17:1558-68. [Crossref] [PubMed]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Gupta A, Love S, Schuh A, et al. DOC-MEK: a double-blind randomized phase II trial of docetaxel with or without selumetinib in wild-type BRAF advanced melanoma. Ann Oncol 2014;25:968-74. [Crossref] [PubMed]
- Middleton M, Hauschild A, Thomson D, et al. Results of a multicenter randomized study to evaluate the safety and efficacy of combined immunotherapy with interleukin-2, interferon-{alpha}2b and histamine dihydrochloride versus dacarbazine in patients with stage IV melanoma. Ann Oncol 2007;18:1691-7. [Crossref] [PubMed]
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012;380:358-65. [Crossref] [PubMed]
- Hauschild A, Garbe C, Stolz W, et al. Dacarbazine and interferon alpha with or without interleukin 2 in metastatic melanoma: a randomized phase III multicentre trial of the Dermatologic Cooperative Oncology Group (DeCOG). Br J Cancer 2001;84:1036-42. [Crossref] [PubMed]
- Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 2012;367:1694-703. [Crossref] [PubMed]
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 2012;367:107-14. [Crossref] [PubMed]
- Dorval T, Negrier S, Chevreau C, et al. Randomized trial of treatment with cisplatin and interleukin-2 either alone or in combination with interferon-alpha-2a in patients with metastatic melanoma: a Federation Nationale des Centres de Lutte Contre le Cancer Multicenter, parallel study. Cancer 1999;85:1060-6. [Crossref] [PubMed]
- Bajetta E, Del Vecchio M, Nova P, et al. Multicenter phase III randomized trial of polychemotherapy (CVD regimen) versus the same chemotherapy (CT) plus subcutaneous interleukin-2 and interferon-alpha2b in metastatic melanoma. Ann Oncol 2006;17:571-7. [Crossref] [PubMed]
- Atzpodien J, Neuber K, Kamanabrou D, et al. Combination chemotherapy with or without s.c. IL-2 and IFN-alpha: results of a prospectively randomized trial of the Cooperative Advanced Malignant Melanoma Chemoimmunotherapy Group (ACIMM). Br J Cancer 2002;86:179-84. [Crossref] [PubMed]
- Davis LE, Shalin SC, Tackett AJ. Current state of melanoma diagnosis and treatment. Cancer Biol Ther 2019;20:1366-79. [Crossref] [PubMed]
- Bodenham DC. A study of 650 observed malignant melanomas in the South-West region. Ann R Coll Surg Engl 1968;43:218-39. [PubMed]
- Koller KM, Wang W, Schell TD, et al. Malignant melanoma-The cradle of anti-neoplastic immunotherapy. Crit Rev Oncol Hematol 2016;106:25-54. [Crossref] [PubMed]
- Hill GJ 2nd, Krementz ET, Hill HZ. Dimethyl triazeno imidazole carboxamide and combination therapy for melanoma. IV. Late results after complete response to chemotherapy (Central Oncology Group protocols 7130, 7131, and 7131A). Cancer 1984;53:1299-305. [Crossref] [PubMed]
- Chiarion Sileni V, Nortilli R, Aversa SM, et al. Phase II randomized study of dacarbazine, carmustine, cisplatin and tamoxifen versus dacarbazine alone in advanced melanoma patients. Melanoma Res 2001;11:189-96. [Crossref] [PubMed]
- Quirt I, Verma S, Petrella T, et al. Temozolomide for the treatment of metastatic melanoma: a systematic review. Oncologist 2007;12:1114-23. [Crossref] [PubMed]
- Patel PM, Suciu S, Mortier L, et al. Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: final results of a randomised phase III study (EORTC 18032). Eur J Cancer 2011;47:1476-83. [Crossref] [PubMed]
- Chiarion-Sileni V, Guida M, Ridolfi L, et al. Central nervous system failure in melanoma patients: results of a randomised, multicentre phase 3 study of temozolomide- and dacarbazine- based regimens. Br J Cancer 2011;104:1816-21. [Crossref] [PubMed]
- Agarwala SS, Lee SJ, Yip W, et al. Phase III Randomized Study of 4 Weeks of High-Dose Interferon-alpha-2b in Stage T2bNO, T3a-bNO, T4a-bNO, and T1-4N1a-2a (microscopic) Melanoma: A Trial of the Eastern Cooperative Oncology Group-American College of Radiology Imaging Network Cancer Research Group (E1697). J Clin Oncol 2017;35:885-92. [Crossref] [PubMed]
- Atkins MB, Kunkel L, Sznol M, et al. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am 2000;6:S11-4. [PubMed]
- Smith FO, Downey SG, Klapper JA, et al. Treatment of metastatic melanoma using interleukin-2 alone or in conjunction with vaccines. Clin Cancer Res 2008;14:5610-8. [Crossref] [PubMed]
- Bhatia S, Tykodi SS, Thompson JA. Treatment of metastatic melanoma: an overview. Oncology (Williston Park) 2009;23:488-96. [PubMed]
- Schadendorf D, Larkin J, Wolchok J, et al. Health-related quality of life results from the phase III CheckMate 067 study. Eur J Cancer 2017;82:80-91. [Crossref] [PubMed]
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373:23-34. [Crossref] [PubMed]
- Weber JS, D'Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015;16:375-84. [Crossref] [PubMed]
- Hassel JC, Heinzerling L, Aberle J, et al. Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): Evaluation and management of adverse drug reactions. Cancer Treat Rev 2017;57:36-49. [Crossref] [PubMed]
- Long GV, Fung C, Menzies AM, et al. Increased MAPK reactivation in early resistance to dabrafenib/trametinib combination therapy of BRAF-mutant metastatic melanoma. Nat Commun 2014;5:5694. [Crossref] [PubMed]
- Menzies AM, Haydu LE, Carlino MS, et al. Inter- and intra-patient heterogeneity of response and progression to targeted therapy in metastatic melanoma. PLoS One 2014;9:e85004 [Crossref] [PubMed]
- Essner R, Lee JH, Wanek LA, et al. Contemporary surgical treatment of advanced-stage melanoma. Arch Surg 2004;139:961-6; discussion 966-7. [Crossref] [PubMed]
- Meyer T, Merkel S, Goehl J, et al. Surgical therapy for distant metastases of malignant melanoma. Cancer 2000;89:1983-91. [Crossref] [PubMed]
- Deutsch GB, Flaherty DC, Kirchoff DD, et al. Association of Surgical Treatment, Systemic Therapy, and Survival in Patients With Abdominal Visceral Melanoma Metastases, 1965-2014: Relevance of Surgical Cure in the Era of Modern Systemic Therapy. JAMA Surg 2017;152:672-8. [Crossref] [PubMed]
- Feun LG, Gutterman J, Burgess MA, et al. The natural history of resectable metastatic melanoma (Stage IVA melanoma). Cancer 1982;50:1656-63. [Crossref] [PubMed]
- Overett TK, Shiu MH. Surgical treatment of distant metastatic melanoma. Indications and results. Cancer 1985;56:1222-30. [Crossref] [PubMed]
- Wong JH, Euhus DM, Morton DL. Surgical resection for metastatic melanoma to the lung. Arch Surg 1988;123:1091-5. [Crossref] [PubMed]
- Karp NS, Boyd A, DePan HJ, et al. Thoracotomy for metastatic malignant melanoma of the lung. Surgery 1990;107:256-61. [PubMed]
- Gorenstein LA, Putnam JB, Natarajan G, et al. Improved survival after resection of pulmonary metastases from malignant melanoma. Ann Thorac Surg 1991;52:204-10. [Crossref] [PubMed]
- Harpole DH Jr, Johnson CM, Wolfe WG, et al. Analysis of 945 cases of pulmonary metastatic melanoma. J Thorac Cardiovasc Surg 1992;103:743-8; discussion 748-50. [Crossref] [PubMed]
- Tafra L, Dale PS, Wanek LA, et al. Resection and adjuvant immunotherapy for melanoma metastatic to the lung and thorax. J Thorac Cardiovasc Surg 1995;110:119-28; discussion 129. [Crossref] [PubMed]
- Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. [Crossref] [PubMed]
- Leo F, Cagini L, Rocmans P, et al. Lung metastases from melanoma: when is surgical treatment warranted? Br J Cancer 2000;83:569-72. [Crossref] [PubMed]
- Dalrymple-Hay MJ, Rome PD, Kennedy C, et al. Pulmonary metastatic melanoma -- the survival benefit associated with positron emission tomography scanning. Eur J Cardiothorac Surg 2002;21:611-4; discussion 614-5. [Crossref] [PubMed]
- Andrews S, Robinson L, Cantor A, et al. Survival after surgical resection of isolated pulmonary metastases from malignant melanoma. Cancer Control 2006;13:218-23. [Crossref] [PubMed]
- Petersen RP, Hanish SI, Haney JC, et al. Improved survival with pulmonary metastasectomy: an analysis of 1720 patients with pulmonary metastatic melanoma. J Thorac Cardiovasc Surg 2007;133:104-10. [Crossref] [PubMed]
- Neuman HB, Patel A, Hanlon C, et al. Stage-IV melanoma and pulmonary metastases: factors predictive of survival. Ann Surg Oncol 2007;14:2847-53. [Crossref] [PubMed]
- Chua TC, Scolyer RA, Kennedy CW, et al. Surgical management of melanoma lung metastasis: an analysis of survival outcomes in 292 consecutive patients. Ann Surg Oncol 2012;19:1774-81. [Crossref] [PubMed]
- Essner R. Surgical treatment of malignant melanoma. Surg Clin North Am 2003;83:109-56. [Crossref] [PubMed]
- Ollila DW, Stern SL, Morton DL. Tumor doubling time: a selection factor for pulmonary resection of metastatic melanoma. J Surg Oncol 1998;69:206-11. [Crossref] [PubMed]
- Raigani S, Cohen S, Boland GM. The Role of Surgery for Melanoma in an Era of Effective Systemic Therapy. Curr Oncol Rep 2017;19:17. [Crossref] [PubMed]
- Long GV, Grob JJ, Nathan P, et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: a pooled analysis of individual patient data from randomised trials. Lancet Oncol 2016;17:1743-54. [Crossref] [PubMed]
- He M, Lovell J, Ng BL, et al. Post-operative survival following metastasectomy for patients receiving BRAF inhibitor therapy is associated with duration of pre-operative treatment and elective indication. J Surg Oncol 2015;111:980-4. [Crossref] [PubMed]


