Repositioning of migrated self-expanding metallic tracheobronchial stent: predictors of a successful maneuver and its impact on survival
Introduction
Endobronchial stents are used to treat airway obstruction due to both malignant and benign obstructive diseases of the trachea or bronchi (1,2). In conjunction with the standard management for the underlying primary disease, stents relieve dyspnea and improve overall functional status (3). Stents comes in various shapes and sizes, are made of a wide variety of biocompatible materials, each with unique insertion techniques (4-6). However, the search for the perfect stent remains elusive (7). The heterogeneity of the underlying obstruction—shape, form and etiology and the unique attributes of each of these stents—their tensile strength, thickness, insertion technique, do not allow for a single stent type to be ideal for all situations. Stents are foreign objects and are not bereft of complications (6). These include malposition, stent-fractures stent-migration, airway-perforation, excessive granulation-tissue formation, hemorrhage, bacterial colonization resulting in stent-associated infections, stent obstructions by tumor, granulation tissue or due to mucostasis (8-12). These complications may be life-threatening and hence may justify surveillance bronchoscopy at an interval of 4–6 weeks after placement (12). Migration of a tracheobronchial stent has been reported to occur in several studies (12-15). For our study, we identified stent migration as situations where the final location of the stent was different from its intended location but also produced a suboptimal effect on restoration of airway patency or the specific intended use of the stent. Some studies report migration rates between 20–50% (6,13), whereas others report a much lower rate (<5%) (14). Migration of stents appears to occur more frequently with hybrid stents (12,16) although the others disagree (17,18), in benign strictures (6), with undersized stents (19) and with tubular stents compared to Y-stents (6). A mismatch between the size of the airway and the stent diameter is thought to be one of the factors resulting in stent migration (20). Migrated stents can be repositioned (14). If unsuccessful, such migrated stents may need to be removed. Few studies discuss the technique and impact of such stent repositioning maneuvers (21).
We reviewed our experience with stent repositioning maneuvers at the University of Florida hospital. Repositioning of self-expanding metallic stents (SEMS) and silicone stents entail different techniques. We reviewed our experience and results with SEMS and attempted to determine—(I) factors related to successful bronchial stent repositioning and (II) determine if the outcome of the repositioning maneuver’s (successful repositioning vs. unsuccessful repositioning) impact on subsequent survival.
Methods
This was a retrospective study that included patients who underwent stent repositioning at the University of Florida hospital between December 2011 and December 2017 were included for this study. Bronchoscopies were done after suspicion for migrated stent was raised based on clinical factors or if there was a concern on a radiographic study. This study was approved by the Institutional Review Board of the University of Florida (No. 201703393). Informed consent was not considered to be necessary since this was a retrospective analysis with data being collected in an anonymized fashion. Collected data was stored in a password protected repository and no patient identifiers were stored. Only those cases where a third-generation hybrid metallic covered SEMS had been used were included in this study. We extracted demographic information, medical history, and stent repositioning procedure-related information from the electronic health record. Demographic variables included sex and age; medical history variables included history of cancer, lung transplant, etiology of stenosis, radiation treatment, and chemotherapy treatment received in the interval between stenting and the repositioning maneuver. We limited our analysis to third generation fully covered metallic stents—AERO tracheobronchial stent (Merit Medical, South Jordan, Utah) and ULTRAFLEX tracheobronchial stent (Ultraflex; Boston Scientific; Natick, MA) to eliminate the effect of the stent type on results of repositioning. Stent-related characteristics included degree of obstruction (>90, 71–90, 50–70) noted at the time of initial stent placement, number of stent positioning revisions, type of stent migration (distal or proximal), location of stent- trachea (proximal/mid/distal, right mainstem, left mainstem, bronchus intermedius and lobar), stent diameter, stent length, cause of the stenting [stenosis, bronchopleural fistula (BPF)/tracheoesophageal fistula (TEF)/bronchomediastinal fistula, pseudomembranous obstruction in lung transplant patients, hemoptysis, airway malacia and stenosis due to recurrent respiratory papillomatosis], procedural complications, anesthesia/sedation during procedure, type of bronchoscope (flexible or rigid), interval (days) between revisions, and duration (minutes) of the procedure. All repositioning procedures except for three were performed by flexible bronchoscopy under moderate sedation unless patients were already intubated and, in such cases, total intravenous anesthesia (TIVA) was preferred. Three cases (two patients) required rigid bronchoscopy based on proceduralist’s preference. Some patients went on to have multiple distinct repositioning maneuvers and, in such cases, they were included as separate entries. Descriptive statistics were calculated for all measures. Primary outcomes were bronchial stent repositioning success and survival (days until death). As validation of successful repositioning, the durations of successful and failed repositioning procedures were compared using an independent t-test. We excluded entries that were incomplete, if the existing stent could not be repositioned and was removed and/or a new stent had to be placed in its place. Such a procedure was classified as a stent removal and replacement. An unsuccessful stent repositioning maneuver was defined as one where the stent could not be repositioned to its desired location.
Statistical analysis
We first set out to analyze data to identify factors related to bronchial stent repositioning outcome. Putative factors were entered into bivariate analyses (chi-square tests of independence, Fishers exact test, and independent t-tests) with stent repositioning outcome. Then, variables related to repositioning at P<0.20 were combined into a logistic regression model predicting stent repositioning outcome. Variables significant at P<0.05 were retained in the final model. Secondly, in order to identify factors related to survival time in patients with bronchial stents, survival distributions were estimated and depicted graphically. Separate survival curves were estimated to explore the effect of two variables: (I) successful and failed repositioning; (II) medical history group (cancer, transplant, or neither). For each of these groupings, survival curves were compared across strata to assess the statistical significance of observed differences by log rank tests. Additionally, the impact of stent repositioning success on patient survival was tested using a Cox proportional hazards regression model. Age, sex, repositioning success, medical history group, degree of obstruction, number of stent positioning revisions, location of stent, stent diameter, stent length, cause of the stenting (stenosis or others), and interval (days) between revisions were considered as possible predictors. Predictors significant at P<0.05 were retained in the final model. It was not possible to incorporate some predictors because there was insufficient variation among study participants. For example, only 7 of 76 cases had radiation and all but 6 were performed with moderate sedation. The data analysis for this study was generated using SAS 9.4 software. Repositioning technique has been described in the supplement.
Results
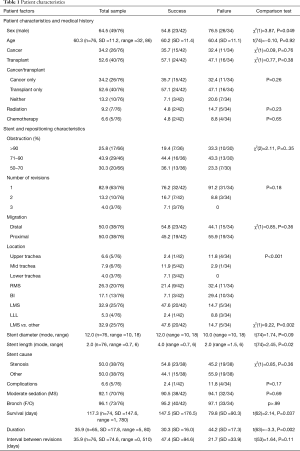
We analyzed 76 (74 Aero, 2 Ultraflex) repositioning maneuvers, of which, 55.3% (n=42) were successful. Six of 26 procedures performed in those with cancer were performed at the time of initial placement. The number was 16 out of 40 procedures for the lung transplant group (P=0.4). There were 21 patients with cancer with four requiring repeated procedures. A total of 24 lung transplant patients underwent repositioning. One patient needed four procedures, three needed three procedures each and seven patients underwent two procedures each. The remaining thirteen required one procedure each. Failed procedures were lengthier than successful cases (30.3 vs. 44.2 minutes; P=0.002). Patient characteristics for the group are described in Table 1.
Full table
Patients were grouped into three categories: cancer (26 procedures/21 patients), lung transplant (40 procedures/24 patients), others neither cancer nor transplant (10 procedures/8 patients). Of these ten procedures, one was done for treatment of post-surgical stenosis of the bronchus intermedius, two for benign tracheal stenosis from polyangiitis with granulomatosis, one for TEF, two for bronchomediastinal fistula, two for recurrent respiratory papillomatosis related airway narrowing and two for airway malacia.
Of the cancer subgroup, 12 were non-small cell cancers (NSCLC), 4 cases were stenosis due to small cell cancers (SCLC), 8 of the procedures were related to TEF from esophageal cancers, one from endobronchial carcinoid tumor related stenosis and 1 from renal cell cancer metastasis. Duration for successful repositioning (mean =30.3 minutes) was significantly shorter than for failures (mean =44.2 minutes), t(63)=−3.3, P=0.002. The probability of success in repositioning procedures was accounted for by patient sex, stent location, and stent diameter (see Table 2).

Full table
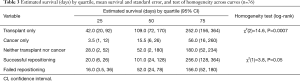
Females were more likely to have a successful repositioning. Stent repositioning in the left main stem (LMS) bronchus was more likely to be successful than stents in other locations, so were stents larger in diameter. Model fit statistics suggest a good fit (AUC =0.78). Cancer and transplant subgroups, degree of obstruction, length of the stent, revision reason i.e., proximal vs. distal migration, interval between initial deployment and revision/revisions, or number of revisions were not related to repositioning success. We tested interactions between medical history group and other covariates (sex, stent diameter, location) to predict repositioning outcome. These interactions were not significant. As shown in Figure 1A, initial survival after stent repositioning is similar for successful and failed repositioning. However, the curves diverge before 100 days and cases with successful repositioning have higher rates of survival. For both groups, the risk of death is high in the first weeks after repositioning. One quarter of cases with successful repositioning were expected to die within 20 days compared to 16 days for failed repositioning (see Table 3).
Full table
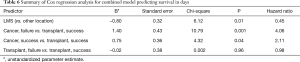
Length of survival diverged for the second quartile: the second quartile of cases were estimated to survive 21–101 days for successful repositioning and 17–52 days for failures. Survival curves estimated for medical history show sharp differences between cancer and transplant cases. For cancer cases, the first quartile was estimated to survived 3.5 days; in comparison, the first quartile of cases with transplantation were estimated to die by day 42 (Table 3). For both groups, the estimated interval for survival for the second quartile of cases widened to 3.51–15.5 days for patients with cancer and 43–109 days for patients with transplants (Table 3). Both stent location, and cancer/transplant subgroup predicted average length of survival (see Table 4).

Full table
Stent location in LMS resulted in a hazard ratio of 0.45, indicating that, on average, cases with LMS located stents were 55% less likely to die than cases with stents in other locations. As expected, transplant cases were 63% less likely to die than cancer cases; cases with neither cancer nor transplant were 29% less likely to die than cancer cases (Figure 1B). However, we did not find the interaction between just the medical history group (cancer vs. transplant) and repositioning outcome to significantly predict length of survival.

We combined two variables—the medical history i.e., cancer vs. transplant, and the success of the stent repositioning maneuver:
- Cancer only, successful repositioning;
- Cancer only, failed repositioning;
- Transplant only, successful repositioning;
- Transplant only, failed repositioning.
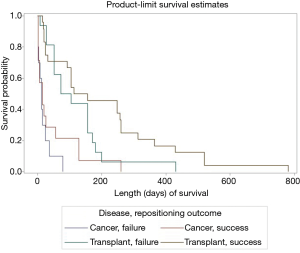
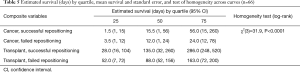
Cases other than with cancer or lung transplant were omitted from this analysis. A survival curve was estimated to explore the effect of this group variable on length of survival. The combined impact of stent repositioning success and medical history on patient survival was tested using a Cox proportional hazards regression model (Figure 2). As shown in Figure 2 and Table 5, initial survival for cancer cases with successful and failed repositioning showed similar survival rate.
Full table
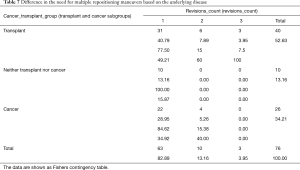
However, cancer cases with successful repositioning survived longer. Specifically, for the third quartile of surviving cancer cases, those with failed repositioning survived an estimated 24 days; cases with successful repositioning survived an estimated 56 days. Although initial survival for transplant cases was slightly higher with failed repositioning, this trend switched for the surviving second and third quartile of cases. Both stent location and medical history/repositioning outcome variables predicted survival (Table 6). All pairwise comparisons were significant except comparison between successful and failed repositioning for transplant cases. No difference in the number of revisions between cancer and transplant groups was found (Fisher’s exact test, P=0.47) (Table 7). Complication rate of 6.5% (5/76) was encountered in this group. One case of major bleeding (>100 cc), two bronchial tears (deployed a balloon expandable iCASTTM stent to cover the breach in one case) and two post-operative respiratory failure requiring intubation and mechanical ventilation were encountered.
Full table
Full table
Discussion
Management of a migrated stent involves either removal followed by replacement if necessary or repositioning of the stent to its desired location. The tendency for stents to migrate differs between different stent types (12,16,17,22). Silicone stents can be fixed to the airway to reduce the possibility of migration but such options exist for SEMS are not available (23). We included only one type of stent in this repositioning study to exclude the effects of different stent types on outcomes. The newer generation AERO stents have built-in features such as anti-migration fins (22), and larger diameter towards the proximal and distal ends (24). To what extent these measures are effective in preventing migration is yet unclear in the absence of studies addressing this specific question. Our study focused on the utility of interventions to reposition these stents and serves a dual purpose—review the result of the repositioning technique and additionally to determine the effectiveness of such maneuvers with focus on survival benefit. A successful procedure was defined as one where the stent could be repositioned to its desired location. Any other outcome was defined as a failure. The stent may have been subsequently removed or left in its suboptimal position with partial restoration of airway patency by other bronchoscopic means. The survival benefit with successful repositioning seen in both lung transplant and cancer subgroup in this study mirror the effect on survival as seen with restoration of airway patency with malignancy (25) and in those with lung transplant related airway complications (13). Clearly, successful repositioning maneuvers are associated with better survival once the early postoperative (<3 weeks) phase is over. We observed a lack of survival benefit with successful repositioning in the initial few days after the procedure for both disease subgroups. Additionally, mortality is unusually high in the first three weeks (quarter of patients with successful repositioning died in the first twenty days after procedure) regardless of the result of the procedure. Restoration of airway patency was not able to provide meaningful survival benefit in the early phase. We doubt this to be secondary to the procedure or technique itself. Rather, this points towards the severity of illness in these patients. Once they were able to survive beyond their initial period of severe sickness; the benefits of a successful repositioning maneuver were clear. In the absence of data to determine the disease-severity index of these patients, this is difficult to prove but remains the most likely explanation. Restoration of airway patency has been known to improve survival in malignant airway obstruction (25) and in cases of anastomotic stenosis among transplant patients (26). The survival benefit from successful stent repositioning maneuver likely emanate from restoration of patency to the central airway. By restoring patency of the central airway, such interventions would reduce the incidence of post-obstructive pneumonia and respiratory failure. Associated improvements in functional status after such an intervention would likely result in these patients receiving treatment for cancer with chemotherapy and/or external beam radiation therapy (25). Such a line of reasoning does not entirely explain the survival benefit effect noted among patients receiving stents for post-transplant anastomotic complications. Murthy et al. (26) report their single center experience with stenting for anastomotic complications. They noted an early survival benefit for patients receiving stents for anastomotic complications. However, this survival benefit appeared to fade over time. We noted no evidence of early survival benefit for those who underwent successful repositioning but recognize a trend towards improved survival later. Early and late survival were defined as survival at 12 and 48 months respectively in Murthy’s study. We however, defined early and later survival for much shorter follow-up periods. Hence the survival benefit noted in our study with successful restoration of airway patency is not different at all from results reported by Murthy et al. This effect results from the restoration of airway patency before long-term stent-related complications may surface. As a matter of practice, we prefer to remove stents as soon as possible and thus may have contributed to the survival benefit seen in our study.
Only three clinical factors were able to predict a successful stent repositioning maneuver—female sex, left mainstem location and larger stent diameters. The left mainstem being longer than its right counterpart allows more length for repositioning and hence may have led to higher success rates. Larger diameter stents are easier to grasp with the forceps and were technically easier to reposition. It remains unclear as to how stent location in the LMS bronchus impacted survival. It also remains unclear as to why female patients were more likely to have a successful procedure. None of the other clinical variables including disease subtype and interval between stenting and repositioning had any impact on survival.
It is thought that stents get epithelialized with time and incite granulation tissue formation both of which would be expected to make repositioning more difficult. We did not experience any variation in the level of difficulty of repositioning with increasing interval between initial placement and repositioning. We believe that the appropriate pretreatment of granulation tissue with ablation may have minimized its impact on subsequent repositioning. Additionally, these third-generation stents are fully covered and minimize the effect of epithelialization. Moreover, some of the late migration events occur due to size mismatch between the stent and the airway so that the stents become loose. This may be due to regression of obstruction with treatment of the malignancy or, in case of lung transplant, resulting from stabilization of the fibrosing process that leads to airway stenosis. Hence, the repositioning maneuver may have become technically less challenging with time and nullified the effect of time interval on repositioning success.
We recognize that the migration events noted in our study form an inhomogeneous group- some of the migration events are immediate migrations/malposition during initial stent placement and others are true delayed migration events over subsequent weeks and months. However, we chose to include all these cases in our final analysis since our intention was to evaluate repositioning technique and not the migration phenomenon itself. The repositioning techniques are the same regardless of the time frame of the migration and the reasons behind it. They are treated the same way. There remains a possibility that the success of the maneuver may have been to some extent impacted by the pathophysiology of the migration. However, the fact that outcomes were not affected by the interval between the initial placement and repositioning goes to provide evidence against such an effect.
We decided to limit the study to only one hybrid stent type to avoid effects of non-uniform stent related factors such as composition, tensile strength, thickness and different repositioning techniques. Third-generation SEMS have different construction methods and composition making them an inhomogeneous group (27).
We were unable to find any large study addressing the topic of stent repositioning. We found one case report that specifically discusses the topic of stent repositioning (21). Our study adds meaningful and crucial data supporting the safety, efficacy and beneficial effect of repositioning maneuvers whenever these are indicated and subsequently performed successfully.
Being a single-center study, it suffers from the lack of external validation. On the other hand, this feature may be also construed as a strength of the study. The procedures were performed by a small number of physicians and hence attest to the uniformity of the technique who had employed strict inclusion criterion.
We feel that whenever applicable an attempt should be made to repositioning the stent if migration is detected either clinically or radiologically or by both. Prompt attention towards this issue is crucial; the need for urgency is highlighted by the high mortality rate seen in the first few days post procedure. This attests to their severity of illness. However, if airway patency is restored by successful repositioning of the stent, these patients may do well over time regardless of their medical diagnosis compared to those cases where repositioning fails. This effect appears to be more pronounced in the cancer subgroup and achieves statistical significance.
Conclusions
Repositioning of migrated stents can be successfully performed regardless of the reasons for initial placement, duration of stenting and degree of original obstruction. Larger stents are easier to reposition and so are stents in the LMS airway. A successful stent repositioning maneuver affected long-term survival for all patients although did not have any impact in the immediate post-procedural period. In general, complication rates are low and manageable.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-608). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board of the University of Florida (No. 201703393). Informed consent was not considered to be necessary since this was a retrospective analysis with data being collected in an anonymized fashion.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wood DE, Liu YH, Vallières E, et al. Airway stenting for malignant and benign tracheobronchial stenosis. Ann Thorac Surg 2003;76:167-72; discussion 173-4. [Crossref] [PubMed]
- Sonett JR, Keenan RJ, Ferson PF, et al. Endobronchial management of benign, malignant, and lung transplantation airway stenoses. Ann Thorac Surg 1995;59:1417-22. [Crossref] [PubMed]
- Ost DE, Ernst A, Grosu HB, et al. Therapeutic bronchoscopy for malignant central airway obstruction: success rates and impact on dyspnea and quality of life. Chest 2015;147:1282-98. [Crossref] [PubMed]
- Lee P, Kupeli E, Mehta AC. Airway stents. Clin Chest Med 2010;31:141-50. Table of Contents. [Crossref] [PubMed]
- Prakash UB. Advances in bronchoscopic procedures. Chest 1999;116:1403-8. [Crossref] [PubMed]
- Bolliger CT, Sutedja TG, Strausz J, et al. Therapeutic bronchoscopy with immediate effect: laser, electrocautery, argon plasma coagulation and stents. Eur Respir J 2006;27:1258-71. [Crossref] [PubMed]
- Hramiec JE, Haasler GB. Tracheal wire stent complications in malacia: implications of position and design. Ann Thorac Surg 1997;63:209-12; discussion 213. [Crossref] [PubMed]
- Colt HG, Dumon JF. Airway stents. Present and future. Clin Chest Med 1995;16:465-78. [PubMed]
- Gaissert HA, Grillo HC, Wright CD, et al. Complication of benign tracheobronchial strictures by self-expanding metal stents. J Thorac Cardiovasc Surg 2003;126:744-7. [Crossref] [PubMed]
- Noppen M, Piérard D, Meysman M, et al. Bacterial colonization of central airways after stenting. Am J Respir Crit Care Med 1999;160:672-7. [Crossref] [PubMed]
- Zakaluzny SA, Lane JD, Mair EA. Complications of tracheobronchial airway stents. Otolaryngol Head Neck Surg 2003;128:478-88. [Crossref] [PubMed]
- Lee HJ, Labaki W, Yu DH, et al. Airway stent complications: the role of follow-up bronchoscopy as a surveillance method. J Thorac Dis 2017;9:4651-9. [Crossref] [PubMed]
- Fernandez-Bussy S, Akindipe O, Kulkarni V, et al. Clinical experience with a new removable tracheobronchial stent in the management of airway complications after lung transplantation. J Heart Lung Transplant 2009;28:683-8. [Crossref] [PubMed]
- Saad CP, Murthy S, Krizmanich G, et al. Self-expandable metallic airway stents and flexible bronchoscopy: long-term outcomes analysis. Chest 2003;124:1993-9. [Crossref] [PubMed]
- Hsu LH, Liu CC, Lin CY, et al. Self-expandable metallic tracheobronchial stent insertion and endobronchial electrocautery with flexible bronchoscopy: preliminary results at a cancer center. J Formos Med Assoc 2002;101:399-405. [PubMed]
- Dooms C, De Keukeleire T, Janssens A, et al. Performance of fully covered self-expanding metallic stents in benign airway strictures. Respiration 2009;77:420-6. [Crossref] [PubMed]
- Chin CS, Litle V, Yun J, et al. Airway stents. Ann Thorac Surg 2008;85:S792-6. [Crossref] [PubMed]
- Noppen M, Stratakos G, D'Haese J, et al. Removal of covered self-expandable metallic airway stents in benign disorders: indications, technique, and outcomes. Chest 2005;127:482-7. [Crossref] [PubMed]
- Ernst A, Herth FJF. Principles and practice of interventional pulmonology. New York: Springer, 2013.
- Stephens KE, Wood DE. Bronchoscopic management of central airway obstruction. J Thorac Cardiovasc Surg 2000;119:289-96. [Crossref] [PubMed]
- Yoneyama R, Saji H, Makino Y, et al. Successful adjustment for self-expanding metallic stent migration using a flexible bronchoscope with two biopsy forceps technique. Gen Thorac Cardiovasc Surg 2017;65:720-3. [Crossref] [PubMed]
- Folch E, Keyes C. Airway stents. Ann Cardiothorac Surg 2018;7:273-83. [Crossref] [PubMed]
- Hohenforst-Schmidt W, Linsmeier B, Zarogoulidis P, et al. Transtracheal single-point stent fixation in posttracheotomy tracheomalacia under cone-beam computer tomography guidance by transmural suturing with the Berci needle - a perspective on a new tool to avoid stent migration of Dumon stents. Ther Clin Risk Manag 2015;11:837-50. [PubMed]
- Mehta AC. AERO self-expanding hybrid stent for airway stenosis. Expert Rev Med Devices 2008;5:553-7. [Crossref] [PubMed]
- Razi SS, Lebovics RS, Schwartz G, et al. Timely airway stenting improves survival in patients with malignant central airway obstruction. Ann Thorac Surg 2010;90:1088-93. [Crossref] [PubMed]
- Murthy SC, Gildea TR, Machuzak MS. Anastomotic airway complications after lung transplantation. Curr Opin Organ Transplant 2010;15:582-7. [Crossref] [PubMed]
- Dutau H, Musani AI, Plojoux J, et al. The use of self-expandable metallic stents in the airways in the adult population. Expert Rev Respir Med 2014;8:179-90. [Crossref] [PubMed]