
Determinants of effective orifice area in aortic valve replacement: anatomic and clinical factors
Introduction
Surgical aortic valve replacement (SAVR) still remains the gold standard therapy to treat patients with severe aortic valve (AV) dysfunction. Prosthetic AV, however, often have small internal orifice area relative to large patient body that may result in a high residual pressure gradient through the prosthetic AV, a phenomenon that is well defined as prosthesis-patient mismatch (PPM) (1-6). A number of studies have shown that small-sized prostheses can be associated with poorer long-term outcomes and survival, as well as a suboptimal regression of the hypertrophied left ventricle (LV), and therefore, current practice guidelines recommend inserting adequately-sized prostheses for SAVR to prevent PPM (4,6-9).
Determining the optimal size of prosthetic AV, however, may not solely depend on the given anatomy of the aortic root, or the surgical condition. Some studies have shown that implanted prosthetic AV size was smaller than expected size by preoperative measurement (10,11). Based on these findings, there may be strong determinant factors which depend on intraoperative settings in SAVR, different from given patient-dependent factors. Moreover, with the advancements of imaging technologies, such as multi-detector computed tomography (MDCT), detailed evaluations of aortic root geometry are now easily available; therefore, the aortic geometric factor can be incorporated to investigate predictors of prosthetic sizes preoperatively (12). In this regard, there have been only a few studies that have provided a comprehensive evaluation of these various factors: including anatomy, co-morbidity, and surgical conditions (1,13).
With this perspective, we sought to determine predictive factors of prosthetic effective orifice area (EOA) in the setting of SAVR, by analyzing clinical, imaging, and surgical parameters.
Methods
Patients, data collection and definitions
Using the Institutional Cardiac Surgical Database of the Asan Medical Center in Seoul, Korea, we identified 1,141 adult patients (≥18 years old) who underwent SAVR between June 2011 and May 2016. The institutional protocol recommended preoperative aortic evaluation using MDCT. Of these patients, we excluded those with active endocarditis or those who underwent concomitant mitral valve surgery (n=242) or annular enlargement procedure for SAVR (n=14). Among the remaining 885 patients, 710 patients [age: 64.9±10.8 years; females: 285 (40.1%)] with preoperative MDCT evaluations on the heart were included in this study, allowing us to obtain detailed parameters for aortic root geometry.
This study was approved by our institutional ethic committee/review board, which waived the requirement for informed consent due to the retrospective nature of the study (IRB: 2019AN0535).
Baseline characteristics, operative profiles, and echocardiographic parameters were primarily retrieved from the institutional electronic database. Further detailed information was obtained by retrospective chart reviews as required.
We created a variable named “surgeon factor”, which consisted of 5 nominal variables corresponding to the five attending surgeons enrolled in this study. Therefore, we expected this variable to represent surgeon-dependent intraoperative conditions, according to individual surgeons. Bovine pericardial bio-prosthesis included Biocor, Magna, Mitroflow, and Trifecta. Supra-annular type prosthesis included Magna, Hancock II, Mosaic, Trifecta, ATS AP360, and Carbomedics TopHat.
Determining postoperative effective orifice area and indexed effective orifice area
Projected EOA of implanted prosthetic valve was calculated using previous published EOA measures corresponding of each valve type and size (in vitro measurement). Mean values for projected EOA were measured using doppler echocardiography in patients with individual valves and were published in papers to use as reference. The reference parameters for each prosthesis model and size are described in Tables S1,S2 (14-17). Indexed EOA (iEOA) was calculated by EOA / Body surface area.
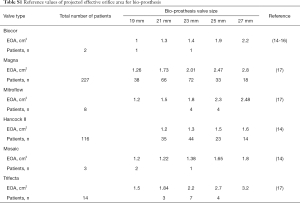
Full table
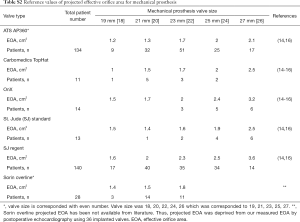
Full table
To evaluate the appropriateness of using projected EOA, measured EOA (which was calculated by echocardiography) was obtained from postoperative 6 to 12 months and used for evaluating the agreement between projected EOA and measured EOA. EOA was calculated using the simplified continuity equation: aortic valve area = [cross-sectional area of left ventricular outflow tract (LVOT) * velocity of LVOT]/velocity of aortic valve (18).
CT acquisition and image analysis
Preoperative MDCT was performed with a second-generation dual-source CT scanner (Somatom Definition Flash; Siemens Medical Solutions, Forchheim, Germany). The need for a CT was determined mainly by the clinician, but cardiac CT examination is generally performed based on the guidelines for the appropriate use of cardiac CT (19,20). Patients with no contraindication to beta-adrenergic blocking agents, and with an initial heart rate exceeding 65 bpm received an oral dose of 2.5 mg of bisoprolol 1 hour before undergoing MDCT. MDCT scanning was conducted in conformance with established guidelines and technical parameters. Using a power injector (Stellant D; Medrad, Indianola, PA, USA), a bolus of 60–80 mL of non-ionic, iodinated contrast material (Iomeron; Bracco Imaging SpA, Milan, Italy) was injected at 4.0 mL/s, followed by 40 mL of a 30:70 mixture of contrast and saline in the guidance of the bolus tracking method (ascending aorta; trigger threshold level 100 HU; scan delay: 8 s). Retrospective electrocardiogram-gated scanning was performed with tube current modulation (dose pulsing windows, 20–70% of the R-R interval). Tube voltage and tube current–time products were adjusted for body size; the scan parameters were as follows: tube voltage = 80–120 kV; tube current = 160–360 mA; pitch = 0.17–0.38; detector collimation = 64×0.6 mm and gantry rotation time =280 ms.
Reconstructed CT datasets with 5% R-R interval were transferred to an external workstation (AquariusNet; TeraRecon, Foster City, CA, USA) for post-processing. CT analysis was independently performed by a cardiac expert who was had no knowledge of clinical findings, including echocardiography findings and operation records. CT image analysis methods for AV evaluation were described in previous articles (21,22). For aortic valve assessment, aortic valve in-plane view (en-face view) was obtained at the systolic phase of CT images. The aortic valve in-plane view is parallel to the transverse plane of the three coronary sinuses and to the images obtained from the aortic root to the LV outflow tract, including the annulus level. Aortic annulus plane was defined as the plane obtained from the nadirs of aortic cusps. Maximal diameter, diameter perpendicular to the maximal diameter, perimeter, and area of aortic annulus were obtained. Maximal diameter of the sinus of Valsalva was obtained from the parallel plane of the annulus level. Maximal diameter of the sinotubular junction was also obtained. Ascending aorta tubular portion diameter was obtained on axial CT image at the level of the right main pulmonary artery (Figure 1).
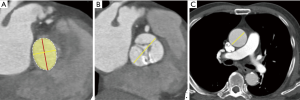
Indexed aortic root parameters were defined as aortic root parameters (aortic valve parameter, diameter of sinus of Valsalva, diameter of sinotubular junction, diameter of aortic tubular portion)/body surface area.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation. Categorical variables are expressed as frequency and percentages. Simple linear regression was used to evaluate the association between iEOA and trans-valvular mean pressure gradient through aortic prosthesis. All baseline parameters were examined in univariable linear regression models to evaluate their association with the iEOA. Then, multivariable linear regression analyses were conducted including only variables with P<0.20 in the univariable models, and a backward elimination method was used to leave only variables with P<0.10 in the final model. We found positive multicollinearity using the variance inflation factor test among aortic annular variables in the linear models. To avoid multicollinearity, only one parameter among all parameters on aortic valve annulus was chosen, based on the highest R2 value in univariable linear regression models, to enter multivariable analyses. To evaluate the appropriateness of using projected EOA, a linear regression analysis was used to examine the relationship between projected and measured EOA. The level of agreement between the two techniques was assessed using Bland-Altman plots and 95% limits of agreement.
All P-values reported were two-tailed, and P≤0.05 was considered as significant. R statistical software (version 3.4.4, R, Vienna, Austria) and SPSS 20(IBM, USA) were used for all data analyses.
Results
Baseline characteristics are described in Table 1. Among the 710 subject patients, 121 patients (17.0%) showed pure aortic insufficiency, while the remaining 589 patients presented with AS with or without insufficiency. Bicuspid aortic valve was present in 331 patients (46.6%), and bio-prosthetic aortic valves were implanted in 370 patients (52.1%), 116 (31.3%) of whom had porcine valves and 254 (69.7%) had pericardial valves. Combined aortic replacement surgery was performed in 104 patients (Table 1).
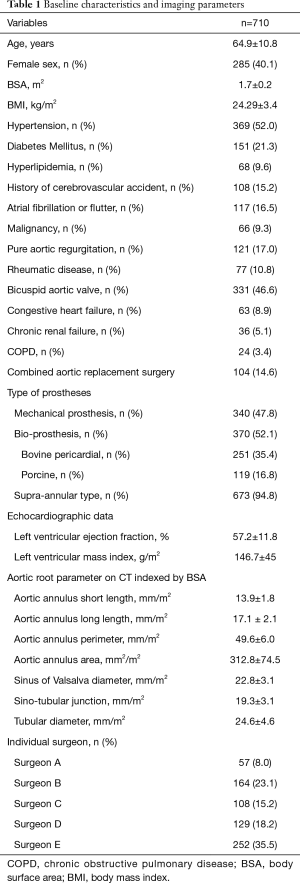
Full table
Mean postoperative iEOA through implanted aortic valve were 1.1±0.3 cm2/m2. There was a significant variation in the iEOA between the five operating surgeons, as shown in Figure 2.

Early mortality (30-day or in-hospital) occurred in 13 patients (1.8%). Postoperative echocardiography was available in 701 patients (98.7%) at a mean of 4.4±2.2 postoperative days. After AVR, mean pressure gradient through prosthetic aortic valve was 15.17±6.17 mmHg. Postoperative iEOA was inversely correlated with mean pressure gradient through prosthetic aortic valve (β=−0.443, R2=0.188, P<0.0001).
Determinants of postoperative iEOA
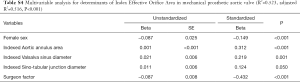
The univariable analysis revealed that a number of variables were significantly associated with iEOA (Table 2). Among aortic annular parameters, indexed aortic valve annulus area that showed the highest R2, and was selected for the multivariable analysis. Using the stepwise technique, the final multivariable model demonstrated that indexed aortic annulus area, indexed Valsalva sinus maximum diameter, male sex, use of bovine pericardial bio-prosthesis, use of supra-annular type prosthesis and surgeon factor showed higher iEOA (adjusted R2=0.513, P<0.001) (Table 3, Figure 3).
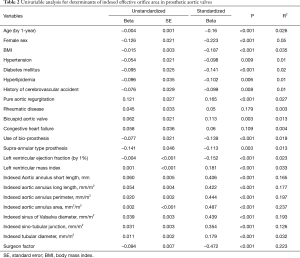
Full table
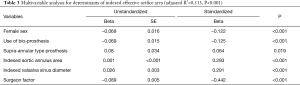
Full table
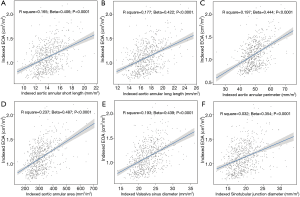
Subgroup analysis
Subgroup analysis was performed based on the types of prostheses: bio-prosthesis and mechanical prosthetic groups (Tables S3 and S4). The multivariable analysis of these subgroups revealed that female sex, indexed annular area, indexed maximal diameter of the sinus and surgeon factors were significant determinants associated with iEOA in both mechanical and bioprosthetic subgroups (P≤0.001, Tables S3 and S4), while there were several additional significant risk variables in each of these groups.
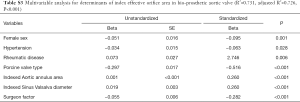
Full table
Full table
Agreement between projected EOA and measured EOA
To evaluate the appropriateness of projected EOA compared with measured EOA, Bland-Altman analysis was performed using measured EOA, between 6–12 months postoperatively, using doppler echocardiography. A total of 64 measured EOA of prosthetic AV were available during this period. Thus, we evaluated the appropriateness between projected and measured EOA in these 64 patients. The linear regression model revealed there was a significant correlation between measured and projected iEOA (β=0.644, R2=0.405, P<0.0001). The Bland-Altman analysis also revealed a good agreement between the two values, with a mean difference of −0.39 cm2/m2 and limits of agreement of 0.43 to −1.21 cm2/m2 [measured IEOA – projected IEOA (measured IEOA + projected EOA)/2].
Discussion
In this study, indexed aortic annular area, indexed maximal diameter of the Valsalva sinus, female sex, and use of bio-prosthesis, supra-annular type prosthesis and surgeon factors emerged as significant determinants of postoperative indexed effective orifice through prosthetic aortic valve in SAVR. Therefore, iEOA was determined not only by a given patient’s characteristics, and but also by surgeon-dependent factors.
The given size of aortic root anatomy is important to decide on an adequate size of prosthetic valve in SAVR. The predictive value of MDCT for determining prosthetic valve sizes in trans-catheter aortic valve replacement (TAVR) has been well established by a number of studies (23,24), and CT can provide reliable and reproducible aortic root parameters in candidates who require aortic valve intervention (11,25,26). In patients receiving SAVR, however, the predictive role of aortic root parameters measured by preoperative CT has been limited, and the decision of which valve size to use has been primarily made in the operative field. Our study intended to evaluate the predictive role of preoperative CT parameters in determining adequate prosthetic valve size in SAVR, as were in TAVR, in association with other clinical factors such as patient profile, type of prosthesis and surgeon factors. As we expected, preoperative CT parameters such as indexed aortic annulus area and indexed diameter of sinus of Valsalva were closely correlated with postoperative iEOA of aortic valve in both univariable and multivariable analyses. Pollari and colleagues stated that CT scan is better method than only echocardiographic investigation in terms of precise aortic root evaluation (27).
Interestingly, the surgeon factor showed a greater coefficient of determination of iEOA. Meanwhile, the estimation and selection of instrument size have been mainly determined in the operative field at the discretion of the surgeon. This sizing process has commonly depended upon the intraoperative manual measurement using the manufacture’s size rather than from preoperative exams derived by objective measurements. With respect to sizing and determination of prosthetic valve in SAVR, there have always been issues where prosthetic valve size would vary depending on surgeon performance, even in similar clinical conditions, such as equivalent anatomical features. Based on our study and prior studies, the surgeon factor may include suture technique, choice of valve type (intra-annular versus supra-annular), and preference of aortic root enlargement technique. Some authors have shown that simple interrupted or continuous suture technique may obtain larger EOA than pledget assisted mattress suture (28,29). Supra-annular type prosthesis had higher EOA than intra-annular type prosthesis (30,31). Moreover, the root enlargement technique is important to avoid taking suboptimal EOA. This association fits well with the practical intuition in that sense of tightness of the prosthesis into the annulus as well as the technical details including other methods—all of which vary with respect to the surgeon—might have an influence on valve sizing (32). Further research is required to examine whether this greater value can be translated into a dominant contribution of the surgeon factor in the determination of prosthesis size, rather than of the anatomic aortic root size of patients. To the best of our knowledge, this is the first study to clearly demonstrate the association between the surgeon factor and postoperative iEOA.
Besides surgeon factor and preoperative CT parameters, female sex and the use of bio-prosthesis were also shown to be predictive for smaller iEOA in the multivariable model. These variables were presented as independent risk factors for PPM in the previous analyses (1,13). In general, mechanical prostheses are known to have a better hemodynamic performance than bio-prosthetic valves when instrument size is equivalent. Accordingly, we believe that for patients with risk factors such as female gender, small aortic root, and candidate for bio-prosthetic valve implantation, prescriptions such as interrupted suture, annular enlargement or use of sutureless valve may be demanded to secure optimal iEOA following SAVR. Sutureless valve achieved higher EOA in similar aortic annular size compared with conventional aortic valve implantation (33).
Limitations
Due to the retrospective nature of the analysis and use of database from a single center, this study may have unmeasured confounding factors and selection bias, even after vigorous statistical adjustment. This study used projected EOA to calculate indexed EOA, instead of measured EOA. In the study of PPM, projected EOA was more reproducible than measured EOA, which is influenced by many clinical factors such as hemodynamic status, poor echocardiographic test, and measurement variation. Among risk factors, surgeon factor is a subjective parameter. However, the significance of the impact of surgeon factor on determining EOA was consistent across various statistical approaches.
Conclusions
Securing optimal iEOA is paramount to improve survival and long-term outcomes in patients who receive SAVR. This study found that female sex, the use of bio-prosthesis, the use of supra-annular type prosthesis, aortic root parameters derived from MDCT, and surgeon factors were significant and independent determinants of iEOA following SAVR. Therefore, besides given individual condition, the surgeon factor is an important factor in determining postoperative iEOA. In patients with small aortic annulus and root size measured by preoperative CT, when associated with other risk factors, active measures before surgery should be considered to secure optimal postoperative iEOA.
Acknowledgments
Funding: This study was supported by a grant (2016-033) from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-188). JBK serves as an unpaid editorial board member of Journal of Thoracic Disease from Feb 2019 to Jan 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by our institutional ethic committee/review board, which waived the requirement for informed consent due to the retrospective nature of the study (IRB: 2019AN0535).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dayan V, Vignolo G, Soca G, et al. Predictors and Outcomes of Prosthesis-Patient Mismatch After Aortic Valve Replacement. JACC Cardiovasc Imaging 2016;9:924-33. [Crossref] [PubMed]
- Takagi H, Yamamoto H, Iwata K, et al. A meta-analysis of effects of prosthesis-patient mismatch after aortic valve replacement on late mortality. Int J Cardiol 2012;159:150-4. [Crossref] [PubMed]
- Head SJ, Mokhles MM, Osnabrugge RL, et al. The impact of prosthesis-patient mismatch on long-term survival after aortic valve replacement: a systematic review and meta-analysis of 34 observational studies comprising 27 186 patients with 133 141 patient-years. Eur Heart J 2012;33:1518-29. [Crossref] [PubMed]
- Chen J, Lin Y, Kang B, et al. Indexed effective orifice area is a significant predictor of higher mid- and long-term mortality rates following aortic valve replacement in patients with prosthesis-patient mismatch. Eur J Cardiothorac Surg 2014;45:234-40. [Crossref] [PubMed]
- Swinkels BM, de Mol BA, Kelder JC, et al. Prosthesis-Patient Mismatch After Aortic Valve Replacement: Effect on Long-Term Survival. Ann Thorac Surg 2016;101:1388-94. [Crossref] [PubMed]
- Une D, Mesana L, Chan V, et al. Clinical Impact of Changes in Left Ventricular Function After Aortic Valve Replacement: Analysis From 3112 Patients. Circulation 2015;132:741-7. [Crossref] [PubMed]
- Flameng W, Rega F, Vercalsteren M, et al. Antimineralization treatment and patient-prosthesis mismatch are major determinants of the onset and incidence of structural valve degeneration in bioprosthetic heart valves. J Thorac Cardiovasc Surg 2014;147:1219-24. [Crossref] [PubMed]
- Mohty D, Dumesnil JG, Echahidi N, et al. Impact of prosthesis-patient mismatch on long-term survival after aortic valve replacement: influence of age, obesity, and left ventricular dysfunction. J Am Coll Cardiol 2009;53:39-47. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:e57-185. [Crossref] [PubMed]
- Wang H, Hanna JM, Ganapathi A, et al. Comparison of aortic annulus size by transesophageal echocardiography and computed tomography angiography with direct surgical measurement. Am J Cardiol 2015;115:1568-73. [Crossref] [PubMed]
- Kempfert J, Van Linden A, Lehmkuhl L, et al. Aortic annulus sizing: echocardiographic versus computed tomography derived measurements in comparison with direct surgical sizing. Eur J Cardiothorac Surg 2012;42:627-33. [Crossref] [PubMed]
- Kasel AM, Cassese S, Bleiziffer S, et al. Standardized imaging for aortic annular sizing: implications for transcatheter valve selection. JACC Cardiovasc Imaging 2013;6:249-62. [Crossref] [PubMed]
- Bonderman D, Graf A, Kammerlander AA, et al. Factors determining patient-prosthesis mismatch after aortic valve replacement--a prospective cohort study. PLoS One 2013;8:e81940. [Crossref] [PubMed]
- Pibarot P, Dumesnil JG. Doppler echocardiographic evaluation of prosthetic valve function. Heart 2012;98:69-78. [Crossref] [PubMed]
- Rajani R, Mukherjee D, Chambers JB. Doppler echocardiography in normally functioning replacement aortic valves: a review of 129 studies. J Heart Valve Dis 2007;16:519-35. [PubMed]
- Zoghbi WA, Chambers JB, Dumesnil JG, et al. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: a report From the American Society of Echocardiography's Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr 2009;22:975-1014. [Crossref] [PubMed]
- Ugur M, Suri RM, Daly RC, et al. Comparison of early hemodynamic performance of 3 aortic valve bioprostheses. J Thorac Cardiovasc Surg 2014;148:1940-6. [Crossref] [PubMed]
- Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr 2009;22:1-23. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;70:252-89. [Crossref] [PubMed]
- ASCI Practice Guideline Working Group, Beck KS, Kim JA, et al. 2017 Multimodality Appropriate Use Criteria for Noninvasive Cardiac Imaging: Expert Consensus of the Asian Society of Cardiovascular Imaging. Korean J Radiol 2017;18:871-80. [Crossref] [PubMed]
- Kang JW, Song HG, Yang DH, et al. Association between bicuspid aortic valve phenotype and patterns of valvular dysfunction and bicuspid aortopathy: comprehensive evaluation using MDCT and echocardiography. JACC Cardiovasc Imaging 2013;6:150-61. [Crossref] [PubMed]
- Koo HJ, Kang JW, Kim JA, et al. Functional classification of aortic regurgitation using cardiac computed tomography: comparison with surgical inspection. Int J Cardiovasc Imaging 2018;34:1295-303. [Crossref] [PubMed]
- Onorati F, D'Errigo P, Grossi C, et al. Effect of severe left ventricular systolic dysfunction on hospital outcome after transcatheter aortic valve implantation or surgical aortic valve replacement: results from a propensity-matched population of the Italian OBSERVANT multicenter study. J Thorac Cardiovasc Surg 2014;147:568-75. [Crossref] [PubMed]
- Pibarot P, Weissman NJ, Stewart WJ, et al. Incidence and sequelae of prosthesis-patient mismatch in transcatheter versus surgical valve replacement in high-risk patients with severe aortic stenosis: a PARTNER trial cohort--a analysis. J Am Coll Cardiol 2014;64:1323-34. [Crossref] [PubMed]
- Philip F, Faza NN, Schoenhagen P, et al. Aortic annulus and root characteristics in severe aortic stenosis due to bicuspid aortic valve and tricuspid aortic valves: implications for transcatheter aortic valve therapies. Catheter Cardiovasc Interv 2015;86:E88-98. [Crossref] [PubMed]
- Margaryan R, Kallushi E, Gilmanov D, et al. Sutureless Aortic Valve Prosthesis Sizing: Estimation and Prediction Using Multidetector-Row Computed Tomography. Innovations (Phila) 2015;10:230-5; discussion 5. [Crossref] [PubMed]
- Pollari F, Fischlein T, Pfeiffer S. Comparison of Surgical and Transcatheter Aortic Valve Prostheses: Size Matters. J Am Coll Cardiol 2019;73:2784. [Crossref] [PubMed]
- Tabata M, Shibayama K, Watanabe H, et al. Simple interrupted suturing increases valve performance after aortic valve replacement with a small supra-annular bioprosthesis. J Thorac Cardiovasc Surg 2014;147:321-5. [Crossref] [PubMed]
- Kim HH, Lee S, Joo HC, et al. Impact of Suture Techniques for Aortic Valve Replacement on Prosthesis-Patient Mismatch. Ann Thorac Surg 2020;109:661-7. [Crossref] [PubMed]
- Badano LP, Pavoni D, Musumeci S, et al. Stented bioprosthetic valve hemodynamics: is the supra-annular implant better than the intra-annular? J Heart Valve Dis 2006;15:238. [PubMed]
- Kim SH, Kim HJ, Kim JB, et al. Supra-annular versus intra-annular prostheses in aortic valve replacement: impact on haemodynamics and clinical outcomes. Interact Cardiovasc Thorac Surg 2019;28:58-64. [Crossref] [PubMed]
- Doenst T, Amorim PA, Al-Alam N, et al. Where is the common sense in aortic valve replacement? A review of hemodynamics and sizing of stented tissue valves. J Thorac Cardiovasc Surg 2011;142:1180-7. [Crossref] [PubMed]
- Shalabi A, Spiegelstein D, Sternik L, et al. Sutureless Versus Stented Valve in Aortic Valve Replacement in Patients With Small Annulus. Ann Thorac Surg 2016;102:118-22. [Crossref] [PubMed]


