
Navigational bronchoscopy: a guide through history, current use, and developing technology
Introduction
Navigational bronchoscopy is a type of technology that can be used to help access difficult to reach lesions within the pulmonary parenchyma. The term was initially synonymous with electromagnetic navigation bronchoscopy (ENB). In contemporary practice, it often encompasses peripheral bronchoscopy that is augmented by a computer-aided system (1). Navigational bronchoscopy has higher diagnostic yields when compared to conventional bronchoscopy; the American College of Chest Physicians recommends that ENB be used to obtain tissue from difficult to reach peripheral lung lesions (2). With the advent of lung cancer screening programs, the management of pulmonary nodules found during screening has come to the forefront of thoracic disease. Over 90% of the nodules found in the National Lung Cancer Screening trial were benign in nature (3). To reduce morbidity, using a minimally invasive technique in an attempt to characterize these nodules is of paramount importance (4). Due to the increased use of cardiac CT scans (for coronary calcium scoring) and abdominal CT scans, the incidence of incidentally found lung nodules has also increased (5). Although found incidentally, these pulmonary nodules have significant clinical and economic ramifications (3).
A variety of navigational bronchoscopy platforms have been developed to address this unmet need. The primary goal of these systems is to assist with the procurement of diagnostic tissue in a minimally invasive and safe fashion. Since the early 2000s, there have been advancements in technology that have increased the accuracy of the navigation and improved user interfaces. This allows the tools to be more adaptable across a variety of user skill levels and clinical settings. In this review, we summarize the history of navigational bronchoscopy and characterize the technology used in the past, active clinical use, ongoing clinical trials, and in the future.
History of navigational bronchoscopy
The first navigational systems were ENB platforms that relied upon the generation of an electromagnetic field around the patient’s body. This allowed for the tracking of a sensor within this field. If the volume within the electromagnetic field could be mapped in a fashion which corresponded with a representative 3D reconstruction of the patients anatomy (using CT imaging), then, in theory, the sensor could be “navigated” through this volume with its position being represented on a virtual 3D map of the lung (6). This contrasts with virtual bronchoscopy, which relies on the chest CT scan to generate a rendering of the airway but does not utilize a positioning function. In simplicity, ENB can be related to traveling with the assistance of a global positioning system, while virtual bronchoscopy can be related to traveling with the assistance of a roadmap.
The history of ENB traces back to the use of electromagnetic tracking devices in neurosurgery, urology, otorhinolaryngology, and cardiology (4,7). Early studies investigated the technology in swine models; Solomon et al. were the first to conduct these studies and publish their results (8,9). Dr. Solomon’s work applied the Biosense Intrabody Navigation System (Biosense Webster Inc., Irvine, CA, USA.); which, was already being used in cardiac electrophysiology and liver disease (transjugular intrahepatic portosystemic shunt procedures) (9). Independent of his work, an Israeli company (superDimension Ltd.) developed similar technology. Its application was in computer gaming; the location sensors were placed on handheld paddles. This technology was subsequently miniaturized so that it could be applied within healthcare. Initially used within cardiac electrophysiology, the technology migrated to intrapulmonary applications and bronchoscopic navigation. This formed the foundation for modern ENB platforms (8). Commercialization of the superDimension™ system (Medtronic, Minneapolis, MN, U.S.A.) led to the wide adoption of ENB. The field of navigational bronchoscopy accelerated from this critical junction.
The first human trial using ENB was published in 2006 (10). Since then, dozens of studies have been published utilizing ENB, most of which using a superDimension™ platform. During this time period, many technological advances to registration algorithms, user interfaces, and tools have been implemented. These advances were applied both within the superDimension™ platform and with other ENB-based systems, such as the SPiN Thoracic Navigation System™ by Veran Medical Technologies™ (St. Louis, MO, USA). Electromagnetic navigation is not limited solely to pure ENB systems; some models of contemporary robotic-assisted bronchoscopy platforms also utilize electromagnetic guidance within their workflows (6). Additionally, a variety of non-ENB guidance platforms have also been developed: such as LUNGVISION™ (Body Vision Medical Ltd., Ramat Ha Sharon, Israel) and Archimedes™ by Broncus Medical© (San Jose, CA, USA). LUNGVISION™ uses augmented fluoroscopic and C-arm based tomography to achieve navigation to and localization of peripheral lung nodules, respectively. The Archimedes™ platform uses fluoroscopic guidance as part of its bronchoscopic transparenchymal nodule access procedure.
Over the past two decades, there have been tremendous technological innovations in navigational bronchoscopy. This has led to the availability of a variety of approaches and platforms for navigating to and successfully sampling pulmonary lesions.
Technology in current clinical practice
Interpretation of each technology’s performance in the medical literature is challenging, and often confounded by bias. Given the many iterations of technological advancements and improvements in user interface, it is difficult to generalize the results of prior studies that used earlier-generation software and catheters. Importantly, there are no head-to-head human trials that have assessed competing navigational platforms or technologies. Anecdotally, there appear to be performance differences among the platforms that depend on a variety of characteristics: lesion size, lesion location, patient’s body habitus, etc., though this has yet to be proven in a well-designed clinical trial. Given the capital expense of these technologies, it is unlikely that a head-to-head comparison would have a significant impact on clinical practice. However, understanding the performance advantages and limitations of each individual technology is of paramount importance, with meaningful clinical consequences.
Platform-independent factors that can affect yield do appear to exist. For instance, it has been shown that diagnostic yields are lower when navigational systems used in isolation. The use of adjunctive imaging modalities such as fluoroscopy or radial probe endobronchial ultrasound (RP-EBUS) has been proven to increase diagnostic yield (11). Factors such as lesion location, lesion size, presence of a bronchus sign, anesthesia strategy, and registration error also have significant implications (12). It is likely that other non-technological factors such as user experience and expertise, as well as experience with patient selection, contribute to performance as well (12).
As detailed in the following section, there are a variety of platforms to choose from. Knowledge of their fundamental principles and available data is essential to successful use.
Traditional navigation platforms
superDimension
The superDimension™ system is a well-known ENB system. It was initially released in 2004 and has undergone several series of hardware and software upgrades since. At present, it is the most widely used ENB system. Over 90% of all ENB procedures are performed using this platform (13). Like most other electromagnetic navigation systems, a recent chest CT scan is needed for the planning. superDimension™ recommends a specific CT scan acquisition protocol, highlighted by a slice thickness between 1.0–1.25 mm and a slice interval range between 0.8–1.0 mm (14).
Early studies were primarily single-center case series and varied significantly in their design and outcomes. Since its inception, there have been numerous published studies describing the diagnostic yield using the superDimension™ system. They are summarized in Table 1 (15).
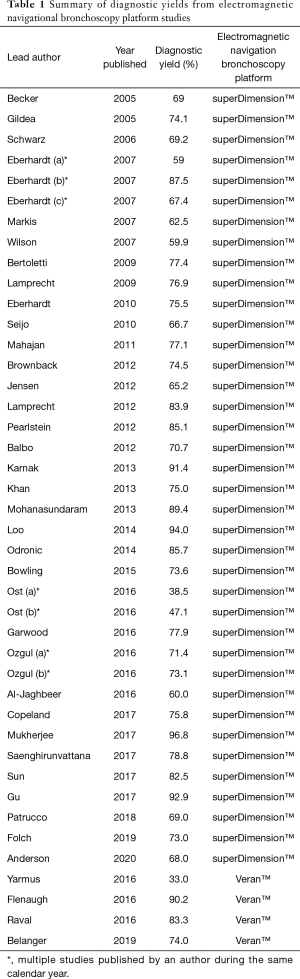
Full table
The superDimension™ platform was recently evaluated in a large, prospective, multicenter study (NAVIGATE). The primary focus of the NAVIGATE study was to evaluate the safety profile of navigational bronchoscopy for peripheral lung lesions (16). Many other outcomes were reported, including diagnostic yield. This prospective, multicenter observational cohort study included over 1,000 patients from 29 centers in the United States. These centers were a mix of both academic and community-based healthcare facilities. Recruitment at each site was capped to 75 subjects to preserve heterogeneity and limit bias. The main inclusion criteria were patients with peripheral lung lesions who were undergoing ENB. Almost half of all lesions (49.1%) were less than 20mm in size. Fifty-eight percent of lesions were in the upper lobe; the median distance between a lesion and pleura was 9.0 mm. Successful navigation and tissue acquisition rate was 94.4%, the 12-month diagnostic yield was 72.9%, and the 12-month sensitivity for malignancy was 68.8%. In this study, complications rates were low: pneumothorax rate of 4.3%, serious bleeding rate of 1.5%, and respiratory failure rate of 0.4%. Limitations of the study included its single-arm non-randomized design, the potential of patient selection bias, and the potential inability to generalize the results to operators who perform less than five ENB cases per month (16). To date, it is the largest published ENB study (16).
SPiN Thoracic Navigation System™
The SPiN Thoracic Navigation System™ is the ENB platform offered by Veran Medical Technologies™. It has a variety of fundamental differences from the superDimension™. A proposed advantage of the Veran™ system is that the complementary tools (Always-On Tip Tracked® Instruments) house electromagnetic sensors that allow tracking of the instrument position and the target lesion throughout the procedure. This could possibly eliminate the need for fluoroscopy (17). The key differentiating features between the two systems are summarized in Table 2.
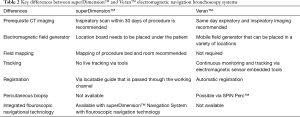
Full table
Complementary to the ENB component, the Veran Medical Technologies™ SPiN Perc™ system also allows a pulmonologist to access nodules percutaneously. The combination of the ENB and SPiN Perc™ components allows for a seamless transition from an endobronchial to a transthoracic needle aspiration during a single procedural setting. A multicenter clinical trial regarding the diagnostic yield of a staged procedure [endobronchial ultrasound transbronchial needle aspiration (EBUS-TBNA), ENB, and electromagnetic navigation-transthoracic needle aspiration (ENM-TTNA)] for the diagnosis of pulmonary nodules is being conducted (18).
There is no head-to-head published data that compares the VeranTM and MedtronicTM ENB systems. In the largest published prospective study of the Veran™ system, a combination of EBUS-TBNA, ENB, and ENM-TTNA were used to assess solitary pulmonary nodules. Overall, the ENB component was diagnostic in 33% of cases; rates rose to 73% when an air bronchus sign was present (19). The combination of ENB and ENM-TTNA yielded a diagnostic rate of 87%. The mean lesion size in this study was 20.3 mm, and the mean distance from the pleura was 12.6 mm. In a retrospective, multicenter study that included 129 ENM-TTNA, a diagnostic rate of 73.7% was achieved with the transthoracic component alone. When combined with ENB, the rate increased to 81.1% (20). The pneumothorax rate was noted to be 17.8%, over half of which needed the placement of a chest tube. In another retrospective study, the diagnostic yield of the ENB component (n=92) was 74% (21). Although these studies were conducted at large academic medical centers, there is data that evaluates the system in other settings. In a community-based practice, the diagnostic rate with the Veran™ ENB system was 78.3% (without a bronchus sign) and 88% (with a bronchus sign) (22).
Archimedes™
Archimedes™ by Broncus Medical© (San Jose, CA, USA) is a virtual bronchoscopic planning and navigational platform. It uses the LungPoint® Virtual Bronchoscopic Navigation System; it is the only virtual bronchoscopic navigation platform currently on the market. The console is designed in a way that a virtual endoscopic map is displayed adjacent to the live endoscopic image. It does not have an electromagnetic navigation component. The system reconstructs the airways and major vessels from the CT scan; it calculates a centerline for each airway. The target lesion is superimposed on the virtual bronchoscopic view. Based on internal testing, the manufacturer states that the user is guided to within 3mm of the target (23). The unique feature of Archimedes™ is that it allows the creation of a tunnel through the lung parenchyma, around blood vessels (bronchoscopic transparenchymal nodule access) (24). In the first-in-human study (n=12), the overall diagnostic yield was 83% (25). Only ten patients were able to have a tunneled path created; biopsy yield was 100% in this group. In order to assess safety and efficacy, all patients who underwent a bronchoscopic biopsy via the creation of a tunneled pathway proceeded to surgical resection immediately after the biopsy. No adverse events were noted during the bronchoscopic procedures. As a follow-up, a prospective feasibility and safety study showed a diagnostic rate of 100% in five patients in which a tunnel was successfully created with the Archimedes™ system. Two patients developed a pneumothorax. In contrast to the previous study, this protocol did not include a surgical resection immediately after the bronchoscopic biopsy was obtained. In a multicenter retrospective analysis, the system was used to evaluate suspicious peripheral pulmonary nodules of 31 patients. The objective of the study was the assessment of procedural performance and safety across both academic and community hospitals. Overall, low procedural complication rates (three in total) and comparable average nodule access times were noted (26).
Novel navigational technology
Fluoroscopic navigation
One explanation for lower yields of bronchoscopic sampling of solitary pulmonary nodules is the divergence between the nodule location on the pre-procedural CT and its actual location during the procedure (27). The use of fluoroscopic navigation that incorporates digital tomosynthesis to re-register the target during the procedure is proposed to help mitigate divergence. The superDimension™ Navigation System with Fluoroscopic Navigation Technology is an example of a combined platform (13). In a study by Aboudara et al., the use of fluoroscopic guidance within ENB procedure resulted in higher yields than conventional ENB (79% vs. 54%, 177 nodules sampled in total) (27).
Similarly, the LUNGVISION™ (Body Vision Medical Ltd, Ramat Ha Sharon, Israel) platform utilizes fluoroscopic imaging in conjunction with artificial intelligence and machine-learning algorithms (28). Research is ongoing, but early clinical trial data shows localization success 85–96% and diagnostic yields of 75–92% (29,30).
Robotic-assisted bronchoscopy
Robotic bronchoscopy is the latest wave of navigational bronchoscopy. At present, there are two platforms that are currently available on the market. Both allow the operator to interact with a bronchoscope via a controller apparatus, which allows precision control.
Auris monarch
The Monarch™ Platform by Auris Health© (Redwood City, CA, USA) is a robotic platform that was approved for use by the United States Food and Drug Administration in March 2018 (31). Rojas-Solano et al. used the system to sample 15 parenchymal lesions (32). In this cohort, a malignant diagnosis was found in nine of the patients; there were no significant adverse events noted. The system was also successfully used to obtain a diagnosis in 97% of nodules (n=77) among eight human cadaveric lungs (33). A retrospective study that assessed the platform across both academic and community medical centers in the United States revealed a diagnostic yield that ranged between 69.1–77%. A multicenter, prospective clinical trial that assesses the successful navigation of the system and the incidence of device or procedure-related adverse effects is currently underway (ClinicalTrials.gov Identifier: NCT03727425) (34).
Intuitive Ion
The Ion™ endoluminal robotic bronchoscopy platform by Intuitive Surgical© (Sunnyvale, CA, USA) is a robotic-assisted bronchoscopy platform that employs the use of fully articulating 3.5mm outer diameter catheter for accessing difficult to reach nodules (35). The working channel for this catheter is 2.0 mm in diameter. The system is designed to allow direct visualization during only a portion of the navigation process. After a specific depth is reached, the visualization probe must be removed. For real-time orientation and feedback, the rest of the navigation process dependent on the system’s shape sensing technology. After reaching the nodule, the catheter is locked into place, and biopsies can be obtained. The catheter allows for 180-degree rotation in all directions. They have a custom-designed needle (Flexision™), which allows sampling around tight radius bends. Additionally, the console is designed in such a way that RP-EBUS, fluoroscopy, and live views of the lung can be presented on a single display. Small studies (29 patients) have shown high rates of reaching the target (96.6% success) and an overall diagnostic yield of 79.3% (88% for malignancy) (36). All the lesions in this study were ≤12.3 mm in size, and 41.4% did not have a bronchus sign present. Another study that evaluated successful navigation rates between ultrathin bronchoscopy with RP-EBUS, ENB, and the Ion™ platform showed that the robotic system outperformed the other modalities with a successful navigation rate of 100% (37). Clinical trials are ongoing (ClinicalTrials.gov Identifier: NCT03893539) to assess the navigation success, biopsy success, and the sensitivity for malignancy from the obtained samples (38).
Cone beam computed tomography (CBCT)
CBCT is an imaging modality that is used frequently by interventional radiologists (39). These systems can reconstruct detailed, isotropic images of a specified anatomical area (40). Unlike traditional multidetector CT, CBCT relies on a high-resolution two-dimensional detector for image acquisition (41). The C-arm of the system needs to be rotated to acquire a 3D data set. Its use with peripheral bronchoscopy is relatively novel; one of the first reported series was published in 2014 (41). Currently, there are a variety of CBCT systems on the market from various manufacturers. It is challenging to classify CBCT under the umbrella of navigations systems; it is rather an adjunctive imaging tool that can help assist with more precise localization of lesions. In simplicity, it can be viewed as a refreshable, static map. As peripheral bronchoscopy is being performed, CBCT can assist with the adjustments to redirect to the lesion, as well as confirm “tool-in-lesion” prior to obtaining biopsy samples. Peripheral bronchoscopy with CBCT can be performed with or without the use of additional navigational bronchoscopy platforms. A study that paired CBCT with thin and ultrathin bronchoscopy showed a diagnostic yield of 70% (40). Although not assessed as a navigational tool, CBCT was used to evaluate the degree of sampling error between the peripheral bronchoscopic modalities in the PRECISION-1 study (37). As CBCT platforms become smaller and more affordable, their use may become more widespread within pulmonary medicine.
Future directions
Data that supports a reduction in lung cancer mortality with screening continues to mount (42). This, in combination with the overuse of chest CT scans, will likely lead to a higher incidence of lung nodule detection (43). For the management of these nodules, it is expected that current technology will continue to advance, and new technology will be developed. One such example of this is the Illumisite™ Platform by Medtronic. Published data is yet to be available; the single platform allows the combination of a variety of guidance and localization techniques to help access a lesion (ENB, fluoroscopic navigation, continuous guidance, and transbronchial access) (44).
Conclusions
In summary, navigational bronchoscopy can be relied upon to reach pulmonary lesions that warrant sampling. There are numerous platforms using unique technology that are currently available on the market. The evidence behind the use of any of these systems, is at best of moderate quality. Most published literature is limited to case series or retrospective studies. When evaluating for high-quality evidence, there is a literature gap in this niche of pulmonary medicine and thoracic oncology. In addition to obtaining a diagnosis for parenchymal lung lesions, precise navigation and localization have other critical clinical applications. Dye marking for thoracic surgery and fiducial placement for stereotactic body radiation therapy both rely on precise navigation and localization (21,45). This precision may also have implications for therapeutic bronchoscopy. Bronchoscopic ablative techniques are currently being studied, accurate localization of lesions will be of paramount importance (46,47). The ceiling has yet to be reached this important and rapidly evolving clinical scenario of thoracic disease.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Fabien Maldonado and Robert Lentz) for the series “Novel diagnostic techniques for lung cancer” published in Journal of Thoracic Disease. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-2019-ndt-11). The series “Novel Diagnostic Techniques for Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. JC reports other from Medtronic, other from BodyVision LTD, outside the submitted work. TRG reports other from Medtronic, other from Intuitive surgical, grants and other from J&J/Ethicon/Auris robotics, outside the submitted work. SKA has no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Khan KA, Nardelli P, Jaeger A, et al. Navigational Bronchoscopy for Early Lung Cancer: A Road to Therapy. Adv Ther 2016;33:580-96. [Crossref] [PubMed]
- Detterbeck FC, Lewis SZ, Diekemper R, et al. Executive Summary: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:7S-37S.
- Lee CI, Tsai EB, Sigal BM, et al. Incidental extracardiac findings at coronary CT: clinical and economic impact. AJR Am J Roentgenol 2010;194:1531-8. [Crossref] [PubMed]
- Franz AM, Haidegger T, Birkfellner W, et al. Electromagnetic tracking in medicine--a review of technology, validation, and applications. IEEE Trans Med Imaging 2014;33:1702-25. [Crossref] [PubMed]
- Yorgun H, Kaya EB, Hazirolan T, et al. Prevalence of incidental pulmonary findings and early follow-up results in patients undergoing dual-source 64-slice computed tomography coronary angiography. J Comput Assist Tomogr 2010;34:296-301. [Crossref] [PubMed]
- Graetzel CF, Sheehy A, Noonan DP. Robotic bronchoscopy drive mode of the Auris Monarch platform. 2019 International Conference on Robotics and Automation (ICRA). Montreal, QC, Canada, 2019:3895-901.
- Ben-Haim SA, Osadchy D, Scnuster I, et al. Nonfluoroscopic, in vivo navigation and mapping technology. Nat Med 1996;2:1393-5. [Crossref] [PubMed]
- Schwarz Y, Mehta AC, Ernst A, et al. Electromagnetic navigation during flexible bronchoscopy. Respiration 2003;70:516-22. [Crossref] [PubMed]
- Solomon SB, White P Jr, Acker DE, et al. Real-time bronchoscope tip localization enables three-dimensional CT image guidance for transbronchial needle aspiration in swine. Chest 1998;114:1405-10. [Crossref] [PubMed]
- Schwarz Y, Greif J, Becker HD, et al. Real-time electromagnetic navigation bronchoscopy to peripheral lung lesions using overlaid CT images: the first human study. Chest 2006;129:988-94. [Crossref] [PubMed]
- Gex G, Pralong JA, Combescure C, et al. Diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules: a systematic review and meta-analysis. Respiration 2014;87:165-76. [Crossref] [PubMed]
- Kalanjeri S, Gildea TR. Electromagnetic Navigational Bronchoscopy for Peripheral Pulmonary Nodules. Thorac Surg Clin 2016;26:203-13. [Crossref] [PubMed]
- Medtronic. superDimension™ Navigation System with Fluoroscopic Navigation Technology - Overview. Accessed 09/29/2019. Available online: https://www.medtronic.com/covidien/en-us/products/interventional-lung-solutions/superdimension-navigation-system.html
- Coviden. Recommended CT Scan and Reconstruction Parameters. Coviden. 2014. Accessed 09/29/2019. Available online: https://www.medtronic.com/content/dam/covidien/library/us/en/product/interventional-lung-solutions/superdimension-navigation-system--recommended-ct-scan-reconstruction-parameters-information-sheet.pdf
- Mehta AC, Hood KL, Schwarz Y, et al. The Evolutional History of Electromagnetic Navigation Bronchoscopy: State of the Art. Chest 2018;154:935-47. [Crossref] [PubMed]
- Folch EE, Pritchett MA, Nead MA, et al. Electromagnetic Navigation Bronchoscopy for Peripheral Pulmonary Lesions: One-Year Results of the Prospective, Multicenter NAVIGATE Study. J Thorac Oncol 2019;14:445-58. [Crossref] [PubMed]
- Technologies VM. Always-On Tip Tracked® Instruments. Veran Medical Technologies. Accessed 09/29/2019. Available online: https://www.veranmedical.com/spin-system/always-on-tip-tracked-instruments/
- Thiboutot J, Lee HJ, Silvestri GA, et al. Study Design and Rationale: A Multicenter, Prospective Trial of Electromagnetic Bronchoscopic and Electromagnetic Transthoracic Navigational Approaches for the Biopsy of Peripheral Pulmonary Nodules (ALL IN ONE Trial). Contemp Clin Trials 2018;71:88-95. [Crossref] [PubMed]
- Yarmus LB, Arias S, Feller-Kopman D, et al. Electromagnetic navigation transthoracic needle aspiration for the diagnosis of pulmonary nodules: a safety and feasibility pilot study. Journal of Thoracic Disease 2016;8:186-94. [PubMed]
- Mallow C, Lee H, Oberg C, et al. Safety and diagnostic performance of pulmonologists performing electromagnetic guided percutaneous lung biopsy (SPiNperc). Respirology 2019;24:453-8. [Crossref] [PubMed]
- Belanger AR, Burks AC, Chambers DM, et al. Peripheral Lung Nodule Diagnosis and Fiducial Marker Placement Using a Novel Tip-Tracked Electromagnetic Navigation Bronchoscopy System. J Bronchology Interv Pulmonol 2019;26:41-8. [Crossref] [PubMed]
- Raval AA, Amir L. Community hospital experience using electromagnetic navigation bronchoscopy system integrating tidal volume computed tomography mapping. Lung Cancer Manag 2016;5:9-19. [Crossref] [PubMed]
- Broncus. LungPoint® Procedure Planning and Navigation System. 2018. Accessed 09/29/2019. Available online: http://www.broncus.com/wp-content/uploads/2018/03/MK-320-LungPoint-Marketing-Brochure_0218.pdf
- Broncus. Archimedes – Total Lung Access Platform. Accessed 09/29/2019. Available online: http://www.broncus.com/products/archimedes/
- Herth FJ, Eberhardt R, Sterman D, et al. Bronchoscopic transparenchymal nodule access (BTPNA): first in human trial of a novel procedure for sampling solitary pulmonary nodules. Thorax 2015;70:326-32. [Crossref] [PubMed]
- Herth FJ, Li S, Sun J, et al. Bronchoscopic TransParenchymal Nodule Access: Evaluation of Safety and Feasibility of Archimedes System A101. ADVANCES IN COUGH, DYSPNEA, AND INTERVENTIONAL PULMONARY. American Thoracic Society International Conference Abstracts: American Thoracic Society; 2017:A7597-A.
- Aboudara M, Roller L, Rickman O, et al. Improved diagnostic yield for lung nodules with digital tomosynthesis-corrected navigational bronchoscopy: Initial experience with a novel adjunct. Respirology 2020;25:206-13. [Crossref] [PubMed]
- Hogarth DK. Use of augmented fluoroscopic imaging during diagnostic bronchoscopy. Future Oncol 2018;14:2247-52. [Crossref] [PubMed]
- Hogarth DK. Accuracy of LungVision augmented reality endobronchial navigation to aid access to solitary pulmonary nodules, confirmed by radial EBUS. The CHEST Annual Meeting; Toronto, Ontario, Canada, 2017.
- Pritchett M. Feasibility of the LungVision augmented endobronchial fluoroscopic navigation and localization system: comparison with cone beam CT for nodule localization. The Chest Annual Meeting; Toronto, Ontario, Canada, 2017.
- Pellegrino K. Auris Health Unveils the FDA-Cleared Monarch Platform, Ushering in a New Era of Medical Intervention. 2018. Accessed 10/16/2019. Available online: https://www.businesswire.com/news/home/20180323005162/en/Auris-Health-Unveils-FDA-Cleared-Monarch-Platform-Ushering
- Rojas-Solano JR, Ugalde-Gamboa L, Machuzak M. Robotic Bronchoscopy for Diagnosis of Suspected Lung Cancer: A Feasibility Study. J Bronchology Interv Pulmonol 2018;25:168-75. [PubMed]
- Chen A, Gildea T, Gillespie C, et al. Robotic-assisted bronchoscopic biopsy of peripheral pulmonary lesions in a cadaveric model with simulated tumor targets. Chest 2018;154:1113A-4A. [Crossref]
- Auris Health I. Robotic Bronchoscopy for Peripheral Pulmonary Lesions. ClinicalTrials.gov. 2018. Accessed 10/13/2019. Available online: https://clinicaltrials.gov/ct2/show/NCT03727425
- Surgical I. Ion by Intuitive. Intuitive Surgical. Accessed 09/30/2019. Available online: https://www.intuitive.com/en-us/products-and-services/ion
- Fielding DIK, Bashirzadeh F, Son JH, et al. First Human Use of a New Robotic-Assisted Fiber Optic Sensing Navigation System for Small Peripheral Pulmonary Nodules. Respiration 2019;98:142-50. [Crossref] [PubMed]
- Yarmus L, Akulian J, Wahidi M, et al. A Prospective Randomized Comparative Study of Three Guided Bronchoscopic Approaches for Investigating Pulmonary Nodules: The PRECISION-1 Study. Chest 2020;157:694-701. [Crossref] [PubMed]
- Surgical I. Clinical Utility for Ion Endoluminal System. ClinicalTrials.gov. Accessed 10/13/2019 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT03893539
- Kwok YM, Irani FG, Tay KH, et al. Effective dose estimates for cone beam computed tomography in interventional radiology. Eur Radiol 2013;23:3197-204. [Crossref] [PubMed]
- Casal RF, Sarkiss M, Jones AK, et al. Cone beam computed tomography-guided thin/ultrathin bronchoscopy for diagnosis of peripheral lung nodules: a prospective pilot study. J Thorac Dis 2018;10:6950-9. [Crossref] [PubMed]
- Hohenforst-Schmidt W, Zarogoulidis P, Vogl T, et al. Cone Beam Computertomography (CBCT) in Interventional Chest Medicine - High Feasibility for Endobronchial Realtime Navigation. J Cancer 2014;5:231-41. [Crossref] [PubMed]
- de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med 2020;382:503-13. [Crossref] [PubMed]
- Schwartz F, Stieltjes B, Szucs-Farkas Z, et al. Over-scanning in chest CT: Comparison of practice among six hospitals and its impact on radiation dose. Eur J Radiol 2018;102:49-54. [Crossref] [PubMed]
- Medtronic. Illumisite™ Platform. Medtronic. 2019. Accessed 03/04/2020. Available online: https://www.medtronic.com/content/dam/covidien/library/us/en/product/interventional-lung-solutions/illumisite-platform-technology-brochure.pdf
- Hyun K, Park IK, Song JW, et al. Electromagnetic navigation bronchoscopic dye marking for localization of small subsolid nodules: Retrospective observational study. Medicine (Baltimore) 2019;98:e14831. [Crossref] [PubMed]
- Safi S, Op den Winkel J, Kramer S, et al. A new bronchoscopic catheter for the transbronchial ablation of pulmonary nodules. Lung Cancer 2018;124:125-9. [Crossref] [PubMed]
- Casal RF, Walsh G, McArthur M, et al. Bronchoscopic Laser Interstitial Thermal Therapy: An Experimental Study in Normal Porcine Lung Parenchyma. J Bronchology Interv Pulmonol 2018;25:322-9. [Crossref] [PubMed]


