Characterising the impact of pneumonia on outcome in non-small cell lung cancer: identifying preventative strategies
Introduction
Lung cancer is the most prevalent malignancy worldwide (1) and the commonest cause of cancer-related death within the UK (2); despite advances in cancer medicine, age-adjusted 1- and 5-year survival remains poor at 32.1% and 9.5% respectively (3). An ageing population and increasing cancer rates, in parallel with advances in diagnostic and anti-cancer therapeutic strategies, are driving growing costs in the field of cancer medicine (4). Since 1995, total health expenditure on cancer has increased by 47.5 billion euros in the European Union and spending on cancer drugs alone has increased by 12.1 billion euros since 2005. A breakdown of these expenditures reveals the high cost of inpatient care, highlighting the importance of reducing unplanned hospital admissions (UHA) (5). In order to achieve this, UHAs in lung cancer need to be better characterised to determine whether there are preventable admissions.
Lung cancer patients often present with pneumonia which remains an important cause of morbidity and mortality during the disease course (6). The pathogenesis of infection is multi-factorial and is influenced by the underlying disease, co-morbidities and iatrogenic immunosuppression. In lung cancer this can range from infections resulting from tumour obstruction of the airways to infective exacerbations of chronic obstructive pulmonary disease (COPD), to opportunistic infections due to immunosuppression (6). The frequency of respiratory infection in UHAs in the existing literature, is between 21.9% and 49% (4,7-9). Studies examining this are largely historical, variable in sample size and may be difficult to extrapolate from given the changes in the epidemiology and treatment of lung cancer. A retrospective study characterising lung cancer patients in the Netherlands highlighted the potential impact of respiratory infection on a nationwide level (10). The burden of community acquired pneumonia is high in cancer in general, with variations by cancer subtype and particularly high rates in lung cancer (11).
Knowing that Lung cancer is a significant global burden, there is strong potential for improving survival if we can identify and treat modifiable risk factors for infection. Specifically, with reference to the uptake of PPSV23 vaccination which is an NHS standard of care vaccine designed to protect against pneumonia and therefore an easily implementable strategy. We hypothesised that pneumonia presents a significant burden and is strongly linked to outcome in lung cancer patients who present to hospital and significantly more so when compared to other cancer subtypes. We carried out an observational study, characterising the impact of pneumonia in three cohorts of patients (see below) and specifically assessing the importance of PPSV23 vaccination in Non-small cell lung cancer (NSCLC) patients.
- NSCLC patients;
- Breast, Colorectal and Prostate cancer patients;
- All other patients without any form of malignancy.
We analysed the proportion of UHAs from a single UK centre within these patient cohorts. NSCLC represents 85% of lung cancer and is pathologically and clinically very different to small cell lung cancer; as a result, this study is limited to the larger NSCLC cohort.
Methods
This retrospective observational analysis was conducted at a single-centre. The University Hospitals Birmingham is one of the largest teaching hospital trusts in England, serving a regional, national and international population and treating almost 2.2 million patients per year. Between April 2016 and June 2018, data was gathered concerning all UHAs to our centre from the entire cohort of NSCLC patients referred to our centre for diagnosis and treatment (n=605); this figure was calculated at the end of our study period and therefore accounts for all new cases of NSCLC found on admission. The time period was dictated by the availability and completeness of data from the electronic patients records (EPR). The study was conducted with the approval of the UHB Trust and registered with the Queen Elizabeth Hospital audit department (audit code 14333).
The total number of NSCLC patients being treated at QEH within our time frame, both in the inpatient and outpatient setting, were identified through an informatics search for the ICD-10 code C349 and cross referenced for accuracy with the EPR ‘prescribing information and communications system’ (PICS), a technique which has shown validity in other studies (12). Our study cohort included all patients with known NSCLC who had a UHA to our centre; we retrospectively ascertained clinical and demographic data at admission and during their inpatient stay in this time period. The data included; demographics, past medication history, 5-year vaccine status (Pneumovax - PPSV23), lung cancer staging [TNM 8th Edition (13)], smoking status and co-morbidities. Smoking status was categorised into current, never smoked and ex-smokers who were further sub-stratified according to those who quit within the last 2 years, and those who quit greater than 2 years ago. This method was employed in an epidemiology study which described how to model smoking history (14). Admission data included haematological, biochemical and microbiological parameters, radiography, treatment initiated upon admission, in-hospital mortality and length of stay as well as 30-day readmission to hospital.
The type of admission was categorised according to those who were admitted with a pneumonia (Pn) and all other admissions (AOA). This was ascertained by scrutinising the presenting complaints from the patient (i.e. “difficulty in breathing”, “shortness of breath”, fever, purulent/productive cough, wheeze, limited exercise capacity) and the working diagnosis as documented in the post-take medical discharge letter. In addition, correlation was made with infection biomarkers (white cell count, neutrophil count, C-reactive protein (CRP), and albumin levels), positive microbiological growth from sputum and blood cultures. All chest radiographs on admission were individually reviewed for evidence of consolidation or pneumonic changes to enrich the clinical data. With cases where there was a degree of ambiguity as to the aetiology of the changes on the “admission chest radiograph”, relevant cases were discussed with radiology colleagues with further review of cross sectional imaging (baseline or most recent CT thorax where available).
Where appropriate, the cause of death was obtained from the medical death certificate and hospital records. Deaths were classified as complications from respiratory infection (e.g., pneumonia, chest sepsis and respiratory or multi-system organ failure secondary to chest sepsis) whether from their initial admission or 30-day readmission, cancer related for those patients who died of disease progression, non-cancer related and if the cause of death was uncertain when records were not available classified as unclear.
A more limited data set were collected, over the same time period as cohort 1, for cohort 2 (Breast, Colorectal or Prostate cancer patients) and cohort 3 (all other patients without any form of malignancy), focusing on the type of UHA (Pn or AOA) and outcome data (length of in-hospital stay and mortality) for inter-cohort comparison.
Statistical analysis
For continuous variables, results are expressed as means and standard deviations and for categorical variables, as counts and percentages. For continuous data, group comparison was carried out using a t-test or Mann-Whitney test depending on the distribution of data. Group differences for categorical data were assessed using the chi squared test of independence. Ordinal data were further assessed using Kendall’s tau-b statistic. Inter-cohort comparison of continuous data was assessed using Kruskal-Wallis analysis. Univariate analyses of risk factors associated with respiratory related UHA were assessed by performing individual unadjusted logistic regression analysis with inclusion of one covariate per model. A backward elimination, stepwise multivariate logistic regression analysis was performed to identify the independent predictors of in-hospital mortality within this dataset as a whole and sub-stratified according to those who presented with a UHA secondary to pneumonia. The tests were considered significant at P<0.05. Missing data were excluded from analyses. All analyses were performed using the IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp. Released 2013. Armonk, NY: IBM Corp. and SAS 9.3 statistical package version (SAS Institute, Inc, Carry, North Carolina, USA).
Results
The informatics search identified 455 NSCLC, 1,190 other cancer and 54,158 non-cancer patient UHAs to be included in analysis. Over the 26-month study period, the UHA rate for patients with NSCLC was 75.2%; there were 455 separate patient UHAs from a total cohort of 605 patients.
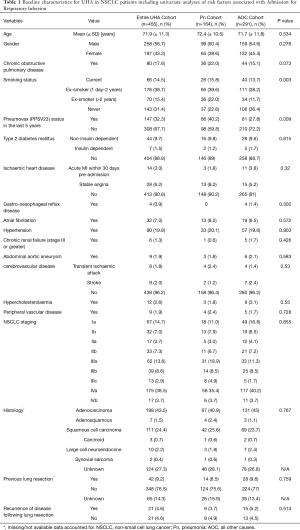
The NSCLC cohort are described in more detail showing that these patients are elderly (mean age 71.9 years) with a small male preponderance (56.7%) and a significant number of co-morbidities including 17.6% of patients with a coded diagnosis of COPD. The NSCLC patient UHAs were further stratified into those that presented with a pneumonia (Pn) and all other admission (AOA) cause (Table 1).
Full table
Within the NSCLC cohort, 164 UHAs were as a direct result of pneumonia (36.0%). The proportion of these UHAs which were associated with a new lung cancer diagnoses following admission was 3.1% (n=14) so 96.9% of UHAs were in known NSCLC patients [64% of new lung cancer diagnoses were stage III disease or higher (n=9)]. Differences in demographic and clinical data between Pn and AOA sub-groups were explored (Tables 1,2); the respiratory cohort were more likely to have had the pure polysaccharide vaccine, Pneumovax (PPSV23) vaccine (P=0.009) and a positive smoking history (P=0.003). Co-morbidities, age; cancer staging and histological diagnosis were not significantly different between the cohorts. Comparison of blood parameters and treatment profiles (Table 2) found CRP to be higher (P=0.001) and albumin to be significantly lower in the Pn sub-group (P<0.0001).

Full table
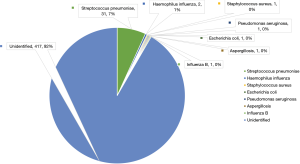
Streptococcus pneumoniae was cultured from the secretions of 31 patients (18.9%) and in 2 cases (1.2%); there was co-growth of Haemophilus influenza (Figure 1). In-hospital mortality (P<0.0001) and length of hospital stay (P=0.031) were significantly higher in the Pn sub-group (Table 3).

Full table
The overall 30-day readmission rate was 5.1% (n=23), 4 from the Pn and 19 from the non-respiratory sub-group (P=0.056). Eight readmissions were due to new pneumonia cases (34.8%). In-hospital mortality following readmission was 65.2% (n=15).
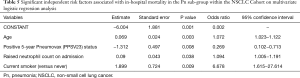
Factors related to in-hospital mortality within the NSCLC patient cohort were further explored by Multivariate analysis (Tables 4,5); patient age, PPSV23 status, Pn admission, Tazocin administration on admission, tumour stages IIb, IIb, IVa and IVb and smoking status were all significant independent predictors of in-hospital mortality. NSCLC staging was also significantly associated with in-hospital mortality on univariate analysis (P=0.017).

Full table
Full table
Within the NSCLC cohort, odds ratios of 0.160 (95% CI 0.077–0.366; P<0.0001) for positive PPSV23 status and 9.522 (95% CI 5.051–17.954; P<0.0001) for Pn status indicate that for patients admitted to hospital with pneumonia without previous PPSV23 in the last 5 years, the odds of death were almost 60-fold higher. There were no significant associations between other blood parameters, tumour histology or comorbidity and in-hospital mortality.
Inter-cohort comparison
There was a significantly higher incidence of pneumonia in the NSCLC cohort (cohort 1) compared with cohorts 2 and 3, P<0.0001 (36.0% versus 1.3% versus 2.2% respectively). Clinical and demographic data for the NSCLC cohort show there was a significantly higher incidence of COPD in the NSCLC cohort compared with cohorts 2 and 3 (P<0.0001).
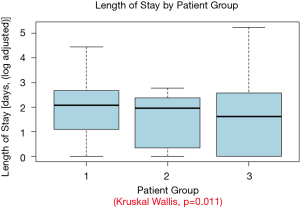
When compared with the outcome data in cohorts 2 and 3, NSCLC patients with pneumonia had a significantly higher rate of in-hospital mortality and length of in-hospital stay compared with pneumonia in cohorts 2 and 3 (P<0.0001 and P=0.011 respectively) (Table 6, Figure 2).

Full table
Discussion
Our study has shown a high incidence of UHA in patients with NSCLC and admission for pneumonia affects over one third of all NSCLC patients admitted to hospital which is higher than for other cancer cohorts (breast, colorectal, prostate) and non-cancer patients. In our NSCLC cohort, we found that 36% of UHAs were due to pneumonia which is similar to 34.3%, a rate from a previously reported study examining bronchoalveolar lavage (BAL) growth in all lung cancer admissions over a retrospective period (7). Similarly, the incidence of community-acquired pneumonia from a population of 89,007 cancer patients revealed 21-fold higher rate in lung cancer patients compared with other non-malignancy, propensity-scored, age-matched cohorts (11). Advanced stage lung cancer in particular is difficult to treat and given the high prevalence of Stage III disease or higher in the respiratory cohort (71.3%, n=117), the control of infection may be an important clinical step in improving the prognosis of lung cancer.
Survival outcome from pneumonia in NSCLC is significantly worse than for other cancer and non-cancer patients. For NSCLC patients, pneumonia is associated with significantly longer lengths of in-hospital stay and is an independent risk factor for in-hospital mortality as is the need for readmission and not having had the PPSV23 pneumonia vaccination. Historical, retrospective data have shown median survival of all lung cancer (small and non-small cell) patients following hospital admission with a respiratory infection was significantly lower than those who did not contract respiratory infection during their inpatient stay (4.2 versus 12.9 months; P<0.05) (15). In the context of a relatively lethal disease such as lung cancer, intervening to reduce the mortality from the tumour itself or from comorbidities is challenging. This study shows that inter-current events such as infection have a detrimental effect on mortality and infection prevention and early management could contribute large gains to overall survival (10,16,17).
The physiological status of patients presenting with pneumonia in the NSCLC cohort was worse than in those who did not. Hypoalbuminaemia is known to be an independent predictor of poor outcome in hospitalised patients, and this association appears to be independent of both inflammation and nutritional status (18). Our study demonstrated that albumin levels on admission to hospital were significantly lower in patients with pneumonia, and CRP levels higher. Neutrophil count emerged as an independent predictor of mortality in the respiratory admission sub-group only; higher counts on admission conferred a poorer in-hospital outcome. This relationship has also been demonstrated in colorectal cancer patients suffering from bacterial infections with higher neutrophil counts conferring poorer cancer-specific survival (P=0.02) (19). In prostate cancer, exposure to medical androgen receptor therapy is associated with a significantly higher risk of community-acquired pneumonia (P<0.001) presumably owing to poorer physiological reserve (20). Chang et al. postulated that prognosis of lung cancer patients with severe pneumonia is mainly dependent on the underlying lung injury, which can be surmised through admission criteria, i.e., higher inflammatory markers connote a more intense systemic inflammatory response indicative of greater on-going parenchymal injury and factors such as previous radiotherapy or chemotherapy also contribute to lung injury (21). Higher neutrophil counts on admission would indicate a robust and active innate immune response with greater degrees of on-going inflammation and injury (22).
Streptococcus pneumoniae was the most frequently cultured organism from sputum in our NSCLC pneumonia sub-group, at a rate of 18.9%. Few studies have managed to address the microbiological repertoire of culprit organisms in pulmonary infection in the setting of NSCLC and culture rates vary considerably. Diagnosing pneumonia in this cohort of patients can prove to be difficult given that clinical signs such as pyrexia and productive cough, allied with raised inflammatory markers such as CRP and white cell count, may be a manifestation of the cancer itself as opposed to pulmonary sepsis. Combining clinical criteria with radiological findings and microbiology is essential. Determining the exact culprit organism in pneumonia is often difficult and unreliable; sputum culture examination has a low specificity and BAL fluid is frequently contaminated with upper airway bacterial commensals (7,8). A 6-year retrospective analysis evaluated 9624 patients with a discharge diagnosis of pneumonia, 46.1% of these patients were culture-negative, of the ones that were culture-positive (51.2%), pathogens that were commonly grown include S. pneumoniae (11.4%), H. influenza (16.7%) and Methicillin Sensitive Staphylococcus Aureus (MSSA) (26.0%) (23). The S. pneumoniae positive cases are potentially vaccine preventable infections. This large study demonstrated that culture-negative pneumonia was independently associated with lower risk for 90-day readmission (23); our study did not demonstrate a significant link between pneumonia and readmission, the numbers were small therefore perhaps not adequately powered to look at this specifically.
This analysis has highlighted the significant difference in mortality between those who have received PPSV23 (3.96%) and those who have not (20.2%). Five-year positive PPSV23 status was significantly higher in the NSCLC Pn sub-group (P=0.009), likely owing to the fact this was a higher risk group and therefore warranted vaccination. Multivariate analysis identified 5-year PPSV23 status as an independent predictor of mortality (P<0.0001); in the entire NSCLC cohort, not having had the vaccine conferred 6.25-fold higher odds of in-hospital death. S. pneumoniae is the most prevalent organism (60–75%) responsible for cases of community acquired pneumonia (CAP) in the UK (24).
In the United Kingdom and in the USA, the administration of PPSV23 is a national health directive led recommendation for all adults above the age of 65 and those below the age of 65 with a long-term health condition. However, for patients with cancer, there has been little investigation into the efficacy of PPSV23. Given that lung cancer is responsible for the highest burden of cancer-related deaths globally, identifying preventable risk factors for early morbidity and mortality in this cohort of patients should be a clear public health aim (1). Preventing pneumonia and reinforcing the need for smoking cessation are crucial. Owing to intensive anti-cancer therapies, lung cancer patients have a degree of immune compromise making them significantly more susceptible to invasive pneumococcal disease (IPD) than the overall adult population (25,26). Studies have shown that between 69% and 72.4% of cancer patient IPD cases were caused by serotypes covered in the 23-valent PPSV23 (26,27). The level of information in the literature regarding the effectiveness of PPSV23 in cancer patients is limited however a nationwide population based cohort study from Taiwan examined the effects of PPSV23 in 157 newly diagnosed elderly lung cancer patients (all comers) (28). Two-year cumulative CAP hospitalisation rates were 37.1% versus 55.4% for lung cancer patients with and without PPSV23 (P<0.001) and overall survival rates were 46.6% versus 26.2% for lung cancer patients with and without PPSV23 (P<0.001) (28).
Our data demonstrated significantly higher antibiotic administration on admission in the NSCLC Pn sub-group; in lung cancer patients in whom infection is suspected, empiric antibiotic therapy is often administered, and more so in the setting of chemotherapy induced neutropenia (6,29). Prophylactic administration of fluoroquinolones post-chemotherapy in lung cancer patients has been shown to reduce the incidence of fever, probable infection and hospitalisation (30). However, advocating increased antibiotic usage in an era of antimicrobial resistance needs challenging. Moreover, specific to NSCLC, in the era of immunotherapy, Microbiome data has demonstrated that the routine use of antibiotics in NSCLC reduces the efficacy of checkpoint blockade agents conferring poorer median overall survival (8.3 months versus 15.3 months, P=0.001) (31).
Conclusions
This study has a number of limitations; firstly it was undertaken in a large tertiary centre in the UK and so it is possible that the UHA rate has been underestimated as patients have attended elsewhere and this study doesn’t include primary care contacts. The study is retrospective making it potentially prone to interpretation or selection bias and the data was not recorded in a systematic, planned manner. A prospective study could have gathered more complete data on performance status and lung function which could contribute to infection risk. More comprehensive data could also then be gathered for NSCLC patients who did not have a UHA to identify risk factors for admission. Similarly, owing to lack of data contemporaneity, it was difficult to perform further sub-group analyses for the “other cancer” and “non-cancer” cohorts as was done for the NSCLC cohort. Despite these limitations this large study does demonstrate the frequency of UHAs for NSCLC patients in a single centre and highlights the importance of pneumonia in the morbidity and mortality of this disease, particularly when compared with other cancer types and indeed non-malignancy patients.
Pneumonia contributes a large burden to the comorbidity of NSCLC. Given that a number of new lung cancer diagnoses were made on admission with pneumonia, being more vigilant and screening for infection in this “at risk” cohort may help to identify disease at an earlier stage and thus bode better patient outcomes. There is a higher burden of pneumonia in NSCLC patients compared with other cohorts, and these patients have a poorer physiological status as demonstrated by our biochemical admission data. This, coupled with poor vaccine uptake in the cohort in general and high antibiotic use in pneumonia patients are highly conducive to poor outcome in this group.Therefore, vigilance for infection, optimising vaccination, judicious use of antibiotics particularly in the era of immunotherapy, patient education, early diagnosis with adequate assessment and efforts to identify a culprit organism should be a priority in both the inpatient and outpatient setting to improve outcome in NSCLC and reduce the incidence of hospital admission. These are all low cost, easy to implement strategies that could be rapidly adopted to make significant advances in reducing mortality from infection in NSCLC.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.04.49). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Clinical patient data was used as part of a clinical audit project (reference 14333) and the study was conducted with the approval of the UHB Trust and registered with the Queen Elizabeth Hospital audit department (audit code 14333).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [Crossref] [PubMed]
- Death registrations summary tables - England and Wales - Office for National Statistics [Internet]. [cited 2018 Jul 31]. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/deathregistrationssummarytablesenglandandwalesreferencetables
- Lung cancer survival statistics [Internet]. Cancer Research UK 2015 [cited 2018 Oct 29]. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer/survival
- Cuppens K, Oyen C, Derweduwen A, et al. Characteristics and outcome of unplanned hospital admissions in patients with lung cancer: a longitudinal tertiary center study. Towards a strategy to reduce the burden. Support Care Cancer 2016;24:2827-35. [PubMed]
- Jönsson B, Hofmarcher T, Lindgren P, et al. The cost and burden of cancer in the European Union 1995-2014. Eur J Cancer 2016;66:162-70. [Crossref] [PubMed]
- Akinosoglou KS, Karkoulias K, Marangos M. Infectious complications in patients with lung cancer. Eur Rev Med Pharmacol Sci 2013;17:8-18. [PubMed]
- Putinati S, Trevisani L, Gualandi M, et al. Pulmonary infections in lung cancer patients at diagnosis. Lung Cancer 1994;11:243-9. [Crossref] [PubMed]
- Kohno S, Koga H, Oka M, et al. The pattern of respiratory infection in patients with lung cancer. Tohoku J Exp Med 1994;173:405-11. [Crossref] [PubMed]
- Berghmans T, Sculier J-P, Klastersky J. A Prospective Study of Infections in Lung Cancer Patients Admitted to the Hospital. Chest 2003;124:114-20. [Crossref] [PubMed]
- Janssen-Heijnen MLG, van Erning FN, De Ruysscher DK, et al. Variation in causes of death in patients with non-small cell lung cancer according to stage and time since diagnosis. Ann Oncol 2015;26:902-7. [Crossref] [PubMed]
- Schmedt N, Heuer OD, Häckl D, et al. Burden of community-acquired pneumonia, predisposing factors and health-care related costs in patients with cancer. BMC Health Serv Res 2019;19:30. [Crossref] [PubMed]
- Nwulu U, Brooks H, Richardson S, et al. Electronic risk assessment for venous thromboembolism: investigating physicians’ rationale for bypassing clinical decision support recommendations. BMJ Open 2014;4:e005647. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Leffondré K, Abrahamowicz M, Siemiatycki J, et al. Modeling Smoking History: A Comparison of Different Approaches. Am J Epidemiol 2002;156:813-23. [Crossref] [PubMed]
- Perlin E, Bang KM, Shah A, et al. The impact of pulmonary infections on the survival of lung cancer patients. Cancer 1990;66:593-6. [Crossref] [PubMed]
- Read WL, Tierney RM, Page NC, et al. Differential Prognostic Impact of Comorbidity. J Clin Oncol 2004;22:3099-103. [Crossref] [PubMed]
- Piccirillo JF, Tierney RM, Costas I, et al. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA 2004;291:2441-7. [Crossref] [PubMed]
- Vincent JL, Dubois MJ, Navickis RJ, et al. Hypoalbuminemia in Acute Illness: Is There a Rationale for Intervention? A Meta-Analysis of Cohort Studies and Controlled Trials. Ann Surg 2003;237:319-34. [Crossref] [PubMed]
- Attiê R, Chinen LTD, Yoshioka EM, et al. Acute bacterial infection negatively impacts cancer specific survival of colorectal cancer patients. World J Gastroenterol 2014;20:13930-5. [Crossref] [PubMed]
- Schmid M, Hanske J, Ravi P, et al. Relationship between androgen deprivation therapy and community-acquired respiratory infections in patients with prostate cancer. Int J Urol 2016;23:305-11. [Crossref] [PubMed]
- Chang Y, Huh JW, Hong SB, et al. Outcomes and prognostic factors of patients with lung cancer and pneumonia-induced respiratory failure in a medical intensive care unit: A single-center study. J Crit Care 2014;29:414-9. [Crossref] [PubMed]
- Hampson P, Dinsdale RJ, et al. Neutrophil Dysfunction, Immature Granulocytes, and Cell-free DNA are Early Biomarkers of Sepsis in Burn-injured Patients: A Prospective Observational Cohort Study. Ann Surg 2017;265:1241-9. [Crossref] [PubMed]
- Andruska A, Micek ST, Shindo Y, et al. Pneumonia Pathogen Characterization Is an Independent Determinant of Hospital Readmission. Chest 2015;148:103-11. [Crossref] [PubMed]
- Lim WS, Baudouin SV, George RC, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax 2009;64 Suppl 3:iii1-55. [Crossref] [PubMed]
- Bartlett JG, Mundy LM. Community-Acquired Pneumonia. N Engl J Med 1995;333:1618-24. [Crossref] [PubMed]
- Wong A, Marrie TJ, Garg S, et al. Increased risk of invasive pneumococcal disease in haematological and solid-organ malignancies. Epidemiol Infect 2010;138:1804-10. [Crossref] [PubMed]
- Garcia-Vidal C, Ardanuy C, Gudiol C, et al. Clinical and microbiological epidemiology of Streptococcus pneumoniae bacteremia in cancer patients. J Infect 2012;65:521-7. [Crossref] [PubMed]
- Chiou WY, Hung SK, Lai CL, et al. Effect of 23-Valent Pneumococcal Polysaccharide Vaccine Inoculated During Anti-Cancer Treatment Period in Elderly Lung Cancer Patients on Community-Acquired Pneumonia Hospitalization: A Nationwide Population-Based Cohort Study. Medicine (Baltimore) 2015;94:e1022. [Crossref] [PubMed]
- Kouranos V, Dimopoulos G, Vassias A, et al. Chemotherapy-induced neutropenia in lung cancer patients: The role of antibiotic prophylaxis. Cancer Lett 2011;313:9-14. [Crossref] [PubMed]
- Cullen M, Gaunt C, Hastings M, et al. Antibacterial Prophylaxis after Chemotherapy for Solid Tumors and Lymphomas. N Engl J Med 2005;353:988-98. [Crossref] [PubMed]
- Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018;359:91-7. [Crossref] [PubMed]