
Hemoptysis complicating ultrasound-guided transthoracic needle lung biopsy: air bronchial sign is a risk predictor
Introduction
Ultrasound-guided transthoracic needle lung biopsy (US-TTLB) is a widespread method to obtain specimens of peripheral pulmonary lesions for pathological examination, with accuracy comparable to that of computed tomography-guided transthoracic needle lung biopsy (CT-TTLB); however, US-TTLB is safer and more efficient, especially for examination of small lesions (1-4). Although US-TTLB is a relatively safe method, it is still an invasive procedure and thus there remains a risk that complications may occur.
Pneumothorax is the most common complication of CT-TTLB, followed by bleeding complications (5,6). In many previously published studies, however, hemoptysis was the most frequent complication of US-guided lung biopsy, followed by pneumothorax (1,7-9). Although past studies have shown that hemoptysis complicating percutaneous transthoracic lung biopsy is typically self-limited, it may be sufficiently severe to terminate the procedure or cause oxygen desaturation, hemodynamic instability, and potential mortality (10). Therefore, it is essential to identify risk factors of hemoptysis to achieve safer transthoracic needle biopsy.
Although previous studies have found that several factors, such as lesion size and open bronchus sign, were associated with hemoptysis after CT-TTLB, the factors influencing the onset of hemoptysis as a complication of US-TTLB are still not fully understood (11-13). Therefore, the aim of this study was to evaluate the incidence of hemoptysis after US-TTLB and to identify related risk factors. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-1247).
Methods
We conducted a retrospective study at Guangzhou Institute of Respiratory Disease. This study was approved by the Scientific Research Ethics Review Committee of the First Affiliated Hospital of Guangzhou Medical University (No. 2018-14). The requirement for written informed consent was waived due to the retrospective design of the study.
Study population
We retrospectively analyzed all data of patients who underwent US-TTLB during the period from February 2013 to December 2016, and only the first procedure of each patients was reviewed. The inclusion criteria were as follows: (I) platelet count was >100,000/mL and prothrombin ratio was <1.4 (14); (II) no anticoagulation or prophylactic hemostasis was used for one week preoperatively; and (III) age was >16 years. The exclusion criteria were as follows: (I) data were missing; (II) the lesion was extrapulmonary; or (III) the core needle cutting biopsy was unused.
Biopsy procedure
With patients placed in the prone, supine, or lateral decubitus position, ultrasound (Esaote Mylab 90, Italy) with a low-frequency (2–5 MHz) convex transducer was used to collect information regarding pulmonary lesions after the clinicians had read the chest computed tomography scan. The biopsy was collaboratively performed by two clinicians with at least 5 years interventional operation experience each; the biopsy plan was then determined by discussion between these operators. Operator1 was sonographer (X Zhou, D Zhou or Q Tang) who evaluated the lesion and was responsible for ultrasound guidance. The distribution of blood flow in each lesion was assessed by Doppler ultrasound. In some cases, contrast-enhanced ultrasound was also used to assess lesions. The contrast agent (Sonovue®, Bracco, Milan, Italy) was injected through a peripheral venous line. Operator2 was an experienced pulmonologist who used an 18G automated core cutting needle with a specimen notch of 20 mm (MC1816, Bard Max. Core, Bard Inc., USA) to perform the biopsy with real-time ultrasound guidance. If the lesion was small, the operators coordinated the procedure with the patient’s respiration to ensure accurate biopsy. The tip of the cutting needle was inserted through the guide channel into the chest wall or lesion, following local anesthesia with 2% lidocaine. The operators made puncture pathways as much as possible to avoid intratumoral blood vessels and allowed at least 22-mm biopsy distances to avoid damage to lung tissues. They then instructed the patient to hold their breath while the biopsy gun was fired. The number of punctures depended on the quality of the specimens and the patient’s tolerance. All specimens were immediately fixed in 10% formalin and sent for histopathological examination.
Recording and treatment of hemoptysis
After biopsy, the patient laid on the biopsy side and was monitored for 30 minutes. If no hemoptysis occurred, the patient was returned to the inpatient ward and kept under close observation. However, if hemoptysis occurred immediately, expectoration was encouraged and oxygen was inhaled through nasal catheters. Hemostatic agents (Haemocoagulase Agkistrodon for Injection, Konruns Pharmaceutical Co., Ltd., Beijing, China) were injected in accordance with the patient’s symptoms, such as dyspnea, oxygen desaturation, or if the amount of hemoptysis was relatively large. Hemoptysis was assessed on the basis of nursing records of puncture biopsy and post-biopsy monitoring in the inpatient ward within 72 hours. If the patient had disease-related hemoptysis before biopsy, but no new hemoptysis occurred during the observation period of 30 minutes after puncture, and no increased hemoptysis volume occurred during the monitoring period, it was not defined as hemoptysis due to US-TTLB.
Study variables
Variables were classified into three categories: patient-related factors, biopsy-related factors, and lesion-related factors.
- Patient-related factors: age, sex, smoking history, pulse oxygen saturation (SpO2), laboratory tests, and the presence or absence of emphysema. Laboratory tests included platelet count, prothrombin time (PT) and activated partial thromboplastin time (APTT). Emphysema was defined as the presence of low density or bullae or bubbles in the lobes of the lung where the target lesion is seen on CT scan images.
- Biopsy-related factors: use of contrast agent, number of punctures and different operators. Ultrasound guided interventional procedures were performed by 3 operators with 5-, 10- and 15-year intervention experience, respectively.
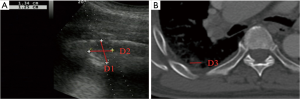
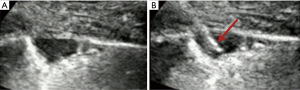
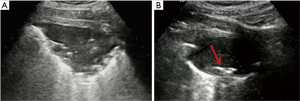
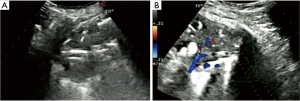
- Lesion-related factors: lesion location (left or right thorax). Lesion size was defined as the maximum diameter of lesion measured in axial computed tomography and divided into size categories of ≤20 and >20 mm (Figure 1). Length of puncture path was defined as the length of the puncture path inside the target lesion measured in ultrasound and was divided into length categories of ≤20 and >20 mm (Figure 1). Lesion pathology was categorized as either benign and malignant in the final clinical diagnosis. The level of air bronchial sign of the lesion was divided into 3 grades: grade 1 = non-air bronchogram (Figure 2); grade 2 = minor air bronchogram (Figure 3); grade 3 = multiple air bronchograms (Figure 4). The grade of air bronchial sign in the puncture section images uploaded on the picture archiving and communication systems were selected for evaluation. If there were fewer punctate, strip or short-arborescent air bronchograms in the lesion, which were not likely to be penetrated by the needle, the ultrasonographic feature of the lesion would be identified as minor air bronchogram. However, if diffuse air bronchograms, multi-strip or arborescent air bronchograms sign were found in the lesion, and the air bronchograms were difficult to avoid penetration by the biopsy needle, the ultrasonographic feature of the lesion would be identified as multiple air bronchograms. Ultrasound images were reviewed by two researchers who had experience in pulmonary ultrasound training. The grade of air bronchogram was assessed by the researchers after ultrasound image review and discussion. If there were still disagreements after discussion between researchers, the grade of air bronchogram would be determined by a sonographer with more than 15 years of experience in thoracic ultrasound. All researchers who assessed the grade of air bronchogram were blinded to clinical details and complications.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows (Version 21.0, IBM Corp., USA). All eventually selected cases were divided into hemoptysis and non-hemoptysis groups, according to the presence or absence of post-biopsy hemoptysis. Continuous variables were expressed as means with standard deviations, and categorical variables were expressed as frequencies or percentages. In univariate analyses, independent two-sample t-tests and chi-squared or Fisher’s exact tests were used as appropriate to compare differences between groups. Multivariate logistic regression analysis was used to identify risk factors of hemoptysis. We also investigated whether incidence of hemoptysis increased according to increased grade of air bronchial sign by the trend test (Mantel-Haenszel test). A P value <0.05 was considered statistically significant.
Results
Characteristics of patients
A total of 221 patients underwent 226 US-TTLBs during the period from February 2013 through December 2016 at the First Affiliated Hospital of Guangzhou Medical University. Among those patients, 12 cases were excluded from the study population because: (I) incomplete information (n=2); (II) extrapulmonary lesions (n=7); (III) age <16 years (n=2), and (IV) fine needle aspiration (n=1). Finally, a total of 214 US-TTLBs on 209 patients were evaluated for this study; five patients underwent two procedures and only the first procedure of each patients was reviewed; thus, 209 US-TTLBs were included (Figure 5). The patients were comprised of 142 men and 67 women, with a mean age of 57.9±14.5 years (range, 17–94 years) and mean platelet count of (278.7±119.5) ×109/L (range, 100×109/L–826×109/L). In our study, 109 patients had history of smoking, and all of them were male patients. Among 209 study patients, there were 72 cases with emphysema in lung diseases, only 2 patients had mild pulmonary hypertension, and 2 patients with pulmonary surgery history. The most common diagnosis was malignant lesions, followed by nonspecific and specific benign lesions; details are shown in Table 1.
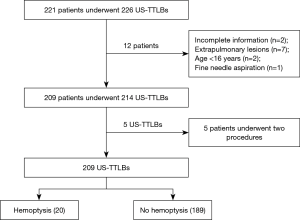
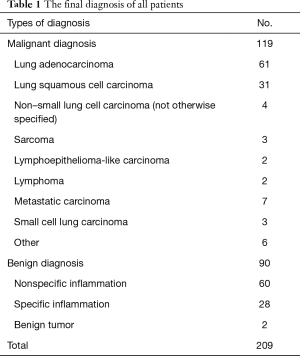
Full table
Frequency of hemoptysis complicating US-TTLBs
Hemoptysis complicating US-TTLB occurred in 20 of the 209 cases (9.6%). Seventeen hemoptysis cases were treated with hemostatic agents. All instances of hemoptysis showed remission or disappeared after termination of puncture and treatment with oxygen and hemostatic agents; the estimated volume of blood loss was 5–20 mL. Another three cases of hemoptysis manifested as phlegm and did not require hemostatic treatment. In addition, there were no cases of hemothorax and none of the patients required surgical hemostasis or died due to hemoptysis complicating US-TTLB.
Logistic regression analyses for hemoptysis
Statistical differences in univariate analyses of the patient-, biopsy-, and lesion-related factors between the hemoptysis group and non-hemoptysis group are shown in Table 2. In univariate analysis, the lesion pathology (P=0.037) and the grade of air bronchial sign (P<0.001) were statistically significant factors between the hemoptysis group and the non-hemoptysis group. However, there were no statistical differences between groups in age, sex, smoking history, SpO2, platelet count, PT, APTT, emphysema, use of contrast agent, number of punctures, experience of operators, lesion location, lesion size, or length of puncture path.
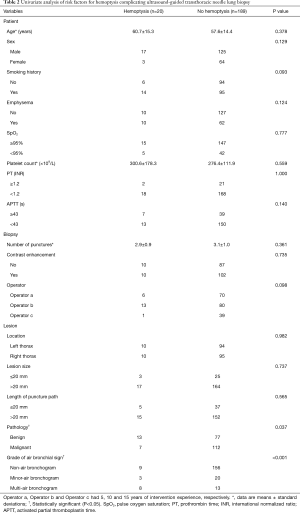
Full table
The results of the multivariate logistic regression analysis to determine independent risk factors for hemoptysis complicating US-TTLB are shown in Table 3. Multivariate analysis showed that the presence of multi-air bronchogram in sonographic image was a statistically significant risk factor for hemoptysis complicating US-TTLB compared with non-air bronchograms (odds ratio =8.946; 95% confidence interval: 2.873–27.863; P<0.001).

Full table
Trend of hemoptysis rate was related to grade of air bronchial sign
The hemoptysis rate complicating US-TTLBs of grade 1 to 3 was 5.5%, 13.0% and 38.1% respectively. There was a statistically significant tendency for incidence of hemoptysis with the grade of air bronchial sign (P for trend <0.001).
Discussion
To the best of our knowledge, this is the first study to investigate risk factors of hemoptysis complicating US-TTLB. Importantly, the study results further proved that US-TTLB was a safe biopsy method and showed that the presence of air bronchograms in an ultrasound scan was a significant predictor of hemoptysis.
Previous studies involving US-TTLB have reported hemoptysis rates of 0.5–22% (1-3,7-9,15). In the present study, the rate of hemoptysis complicating US-TTLB was 9.6%, which was within the overall range of prior publications. However, our reported rate of hemoptysis complicating US-TTLB was higher than that of some previous studies (1-3,7,15). We presumed that we may have recorded hemoptysis in greater detail than those studies, since we recorded hemoptysis in the postoperative ward and included blood sputum and minimal hemoptysis in our assessment. In the largest report of US-TTLB, Guo et al. also recorded the occurrence of hemoptysis in detail and reported a rate of 8.0%, similar to that of our study (8). Furthermore, since we had little acquaintance with the relationship between air bronchogram and hemoptysis in the early time, the puncture pathways were not consciously avoided air-bronchogram in many cases. Therefore, we considered that it was the another reason for the high rate of hemoptysis in our study. In the application of hemostatic drugs, 8.1% (17/209) of cases were treated by hemostatic agents in our study, which was more frequent than the rate in previous studies (1-3,7-9,15). Although the hemoptysis rate reported by Guo et al. was similar to ours, their application rate of drug hemostasis was only 4.9% (8). We presumed that a more conservative treatment strategy in our study might have contributed to the more frequent use of hemostatic medications. However, there were also no cases of hemothorax and none of the patients needed surgical hemostasis in our study. Therefore, this study provides further evidence that US-TTLB is a safe method for the diagnosis of pulmonary lesions.
Air bronchogram in ultrasound scans, particularly dynamic air bronchogram, has been generally used as a common ultrasound imaging sign in the identification of pulmonary lesions (16-18). However, there have been few reports regarding the relationship between air bronchogram and US-TTLB. To the best of our knowledge, this is the first article to discuss the relationship between air bronchogram in ultrasound and postoperative complications after US-TTLB. In a previous study, Kim et al. found that an open bronchus sign on CT was an independent predictor of hemoptysis after CT-TTLB because of the direct connection between the open bronchus and the central airway; hemoptysis occurred when intratumoral hemorrhage occurred during puncture of the hollow open bronchus (13). In ultrasound scans, with the exception of a few residual gas-filled bronchi in the pulmonary lesion, we also speculate that a portion of the air bronchogram sign in ultrasound represents the unobstructed intralesional airway connected to the central airway. Intratumoral bleeding is likely to flow into the central airway through a peripheral unobstructed airway, which manifests as an air bronchogram sign in ultrasound scan. In the present study, there were 21.1% cases (44/209) of air bronchogram. We divided them into non-air bronchogram, minor-air bronchogram and multi-air bronchogram based on the degree of air bronchogram. The rate of hemoptysis in grades 1 to 3 was 5.5%, 13.0% and 38.1% respectively. A statistically significant trend was found in the hemoptysis rate with the grade of air bronchial sign. Therefore, this finding confirmed that additional air bronchial signs in lesions indicate more channels are connected to the central airway and hemoptysis is more likely to occur after US-TTLB. However, although a significant trend between hemoptysis and the grade of air bronchial sign was found, we observed that, in the present study, multivariate analysis showed no significant increase in the rate of hemoptysis among biopsies in lesions with a minor-air bronchogram sign compared with lesions without the air bronchogram sign. Only the presence of multi-air bronchogram in target lesions was found to be a statistically significant ultrasound predictive sign of hemoptysis complicating US-TTLB, when compared with biopsy in lesions without the air bronchogram sign. We considered the reasons are as follows. The biopsy incision in the pulmonary lesion is inevitably accompanied by varying degrees of incision injuries of bronchovascular structures with variable diameter (10). Of special note, generally, injuries of large bronchovascular may be avoided with help of ultrasound real-time guidance, only the minor intratumoral bleeding occur during US-TTLB. In addition, the target lesions in US-TTLBs were subpleural and far away from the hilum. Therefore, these minor intratumoral hemorrhage in subpleural peripheral lesions require adequate channels to flow into the central airway in order to give rise to hemoptysis.
In summary, more bronchial air signs indicate higher the possibility of hemoptysis, while hemoptysis episodes only occurred when the number of bronchial air signs reaches a certain threshold. Fortunately, our present study may provide an ultrasound image indicator for preoperative decision-making in US-TTLB. Target lesion without air bronchogram is a safety sign under ultrasound detection, minor bronchogram normally means relatively low-risk, while multiple bronchogram is a highly dangerous sign of hemoptysis. Operators should be vigilant regarding the occurrence of hemoptysis when an ultrasound scan shows air bronchogram in a pulmonary lesion, and proactively prepare for its treatment, even preoperative use of drugs, in some cases, should be considered in multi-air bronchogram lesions biopsy. However, absence of the air bronchogram sign suggests that operators can place greater focus on accuracy or other risk factors in most cases.
In addition to air bronchogram, we tried to find other variables affecting hemoptysis complicating US-TTLB. Among the variables included in present study, the lesion size, emphysema, gender and APTT were previously reported as predictors of hemoptysis complicating CT-TTLB (11,12,19,20). However, in the present study, except for the air bronchogram sign, no variables showed significant correlations with hemoptysis complicating US-TTLB. Regarding the size of the lesion, Kim et al. explained that the rate of bronchovascular injury may be higher for small lesions because these require more frequent needle redirection during lesion targeting (10). Furthermore, some studies reported the significantly higher bleeding rate after CT-TTLB when the lesion size was less than the throw-length of most cutting needle (12,21,22). Because the tip of the biopsy needle was more likely to extend outside the lesion and damaged normal lung tissue (12,21,22). However, in the present study, there were no significant increases in hemoptysis rate due to the length of puncture path or lesion size when the throw-length (20 mm) of the biopsy needle was used as the grouping factor. Because, for the percutaneous transthoracic biopsy of peripheral lung lesions, ultrasound guidance is safer than computed-tomography guidance, particularly for small lesions (1,3). Assessments of these lesions can benefit from the real-time advantage of ultrasound during biopsy. The operators can cooperate with the patient’s breathing movements and anticipate the safe length of biopsy, thereby reducing the risk of lung tissue damage and avoiding repeated adjustment of the needle tip location. As a controversially discussed predictor for post-biopsy hemoptysis, emphysema is the most common lung comorbidity in the present study. Some previous studies suggested that there was no significant correlation between emphysema and hemoptysis complicating CT-TTLB (10,20,23). However, Yoon et al. found that emphysema was a significant protector against hemoptysis in a multicenter cohort of 10,568 biopsies (11). In the present study, there were no statistical difference in emphysema between hemoptysis and non-hemoptysis groups. And gender may be a confusing factor, because the higher prevalence of subsolid lesions in female patients and higher prevalence of emphysema in male patients (11). Hwang et al. found that prolonged APTT significantly increases hemoptysis, but more than 10% of patients in their study had taken antiplatelets or anticoagulant (20). However, in our study, all treatment of anticoagulation or prophylactic hemostasis were stopped one week preoperatively.
There are limitations in our study. The retrospective nature of the study seems to contribute to the inclusion of confounding factors. For example, in the later stages of the study period, the puncture path may have been consciously chosen to avoid penetration of the air bronchogram, which may have led to bias. Besides, the assessment of air bronchogram in this study was based on a retrospective analysis of static images, such that the relevance between the dynamic air bronchogram and complications could not be assessed. Another limitation is this was a single-center study and the operators who performed puncture and guidance in this study all had extensive experience. Therefore, we cannot fully determine whether this situation is applicable to other centers.
In conclusion, we found that the rate of hemoptysis complicating US-TTLB was 9.6% and the severity of hemoptysis was not serious. Target lesion without air bronchogram is a safety sign, minor bronchogram means relatively low-risk, while multiple bronchogram is a highly dangerous ultrasound sign of hemoptysis.
Acknowledgments
Funding: This work was supported by Science and Technology Planning Project of Guangdong Province, China (grant number 2017A020215062) and Medical Scientific Research Foundation of Guangdong Province, China (grant number A2020408).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1247
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1247
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1247). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Scientific Research Ethics Review Committee of the First Affiliated Hospital of Guangzhou Medical University (No. 2018-14).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yamamoto N, Watanabe T, Yamada K, et al. Efficacy and safety of ultrasound (US) guided percutaneous needle biopsy for peripheral lung or pleural lesion: comparison with computed tomography (CT) guided needle biopsy. J Thorac Dis 2019;11:936-43. [Crossref] [PubMed]
- Jarmakani M, Duguay S, Rust K, et al. Ultrasound Versus Computed Tomographic Guidance for Percutaneous Biopsy of Chest Lesions. J Ultrasound Med 2016;35:1865-72. [Crossref] [PubMed]
- Lee MH, Lubner MG, Hinshaw JL, et al. Ultrasound Guidance Versus CT Guidance for Peripheral Lung Biopsy: Performance According to Lesion Size and Pleural Contact. AJR Am J Roentgenol 2018;210:W110-W117. [Crossref] [PubMed]
- Yuan A, Yang PC, Chang DB, et al. Ultrasound-guided aspiration biopsy of small peripheral pulmonary nodules. Chest 1992;101:926-30. [Crossref] [PubMed]
- Heerink WJ, de Bock GH, de Jonge GJ, et al. Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol 2017;27:138-48. [Crossref] [PubMed]
- DiBardino DM, Yarmus LB, Semaan RW. Transthoracic needle biopsy of the lung. J Thorac Dis 2015;7:S304-16. [PubMed]
- Lei Z, Lou J, Bao L, et al. Contrast-enhanced ultrasound for needle biopsy of central lung cancer with atelectasis. J Med Ultrason 2001;2018:461-7. [PubMed]
- Guo YQ, Liao XH, Li ZX, et al. Ultrasound-Guided Percutaneous Needle Biopsy for Peripheral Pulmonary Lesions: Diagnostic Accuracy and Influencing Factors. Ultrasound Med Biol 2018;44:1003-11. [Crossref] [PubMed]
- Hafez MR, Sobh ES, Elsawy SB, et al. The usefulness of thoracic ultrasonography in diagnosis and staging of bronchogenic carcinoma. Ultrasound 2017;25:200-12. [Crossref] [PubMed]
- Kim H, Kwon D, Yoon SH, et al. Bronchovascular injury associated with clinically significant hemoptysis after CT-guided core biopsy of the lung: Radiologic and histopathologic analysis. PLoS One 2018;13:e0204064. [Crossref] [PubMed]
- Yoon SH, Park CM, Lee KH, et al. Analysis of Complications of Percutaneous Transthoracic Needle Biopsy Using CT-Guidance Modalities In a Multicenter Cohort of 10568 Biopsies. Korean J Radiol 2019;20:323-31. [Crossref] [PubMed]
- Chassagnon G, Gregory J, Al Ahmar M, et al. Risk factors for hemoptysis complicating 17-18 gauge CT-guided transthoracic needle core biopsy: multivariate analysis of 249 procedures. Diagn Interv Radiol 2017;23:347-53. [Crossref] [PubMed]
- Kim H, Park CM, Yoon SH, et al. Open Bronchus Sign on CT: A Risk Factor for Hemoptysis after Percutaneous Transthoracic Biopsy. Korean J Radiol 2018;19:880-7. [Crossref] [PubMed]
- Manhire A, Charig M, Clelland C, et al. Guidelines for radiologically guided lung biopsy. Thorax 2003;58:920-36. [Crossref] [PubMed]
- Yang PC, Chang DB, Yu CJ, et al. Ultrasound-guided core biopsy of thoracic tumors. Am Rev Respir Dis 1992;146:763-7. [Crossref] [PubMed]
- Lichtenstein D, Mezière G, Seitz J. The dynamic air bronchogram. A lung ultrasound sign of alveolar consolidation ruling out atelectasis. Chest 2009;135:1421-5. [Crossref] [PubMed]
- Mongodi S, Via G, Girard M, et al. Lung Ultrasound for Early Diagnosis of Ventilator-Associated Pneumonia. Chest 2016;149:969-80. [Crossref] [PubMed]
- Bugalho A, Ferreira D, Dias SS, et al. The diagnostic value of transthoracic ultrasonographic features in predicting malignancy in undiagnosed pleural effusions: a prospective observational study. Respiration 2014;87:270-8. [Crossref] [PubMed]
- Song YS, Park CM, Park KW, et al. Does antiplatelet therapy increase the risk of hemoptysis during percutaneous transthoracic needle biopsy of a pulmonary lesion? AJR Am J Roentgenol 2013;200:1014-9. [Crossref] [PubMed]
- Hwang EJ, Park CM, Yoon SH, et al. Risk factors for haemoptysis after percutaneous transthoracic needle biopsies in 4,172 cases: Focusing on the effects of enlarged main pulmonary artery diameter. Eur Radiol 2018;28:1410-9. [Crossref] [PubMed]
- Yeow KM, Su IH, Pan KT, et al. Risk factors of pneumothorax and bleeding: multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest 2004;126:748-54. [Crossref] [PubMed]
- Yeow KM, See LC, Lui KW, et al. Risk factors for pneumothorax and bleeding after CT-guided percutaneous coaxial cutting needle biopsy of lung lesions. J Vasc Interv Radiol 2001;12:1305-12. [Crossref] [PubMed]
- Chen CH, Huang WM, Liang SH, et al. Does biopsy needle traversing through central portion of lesion increase the risk of hemoptysis during percutaneous transthoracic needle biopsy? Jpn J Radiol 2018;36:231-7. [Crossref] [PubMed]


