A study on the risk factors of coronary artery disease in patients with Takayasu arteritis
Introduction
Takayasu arteritis (TA) is a chronic, granulomatous, full-thickness arteritis of an unknown etiology. The disease mainly involves the aorta and its main branches, such as the aortic arch, carotid artery, subclavian artery, abdominal aorta, and renal artery. The secondary branches of the aorta, such as the pulmonary artery and coronary artery, can also be involved. Inflammation develops from the outer membrane to the inner membrane leading to thickening, stenosis or occlusion of the involved vascular wall and thrombosis, while the destruction of the elastic layer and muscular layer can lead to aneurysms, dissections, and other diseases (1). TA was first reported by a Japanese ophthalmologist at the 12th Annual Meeting of the Japan Ophthalmology Society held in 1908 in Fukuoka, Mikito Takayasu (2). Arteritis is a systemic disease and its treatment includes medical treatment and surgical treatment. The medical treatment mainly includes anti-inflammatory treatment to control infection, hormone therapy, immunotherapy, vasodilator drugs to improve blood circulation in brain and limb, and antiplatelet drugs, etc. The surgical treatment includes percutaneous endovascular angioplasty and surgical treatment, such as artificial vascular reconstruction, intimal thrombectomy, etc.
The pathogenesis of TA is not clear and is mainly classified as an inflammatory disease. At present, genetic factors, autoimmune cells, and antibody-mediated immune response infections such as Mycobacterium tuberculosis, Chlamydia pneumoniae, and sex hormones may be related to the disease. The main mechanism of TA involvement in coronary artery stenosis is that the inflammation of the aorta extends to the opening and near the segment of the coronary artery, leading to intimal hyperplasia and the fibrosis contracture of the middle and outer membrane causes the stenosis of the lumen. However, there are many studies that suggest that in TA patients with coronary artery disease, it is not simply caused by inflammation (3-5). Previous studies have also shown that (6) the average wall thickness of the ascending aorta in patients with coronary stenosis is thicker than that in patients without coronary stenosis. With the increase of age, the late coronary stenosis may be caused by the contraction of the inflammatory tissue and calcium deposition. There are also studies that show that vascular calcification is not only related to inflammation but also to age and the use of glucocorticoids (7). It has been found that LP (a) in TA patients is significantly increased (8). At present, the relative contribution of specific risk factors is still uncertain, therefore, it is very important to further study and determine the risk factors and clinical characteristics of TA involving coronary artery disease, identify high-risk groups early, and give effective intervention measures early to improve the prognosis of TA patients.
We, therefore, conducted this study to explore the risk factors of TA involvement in coronary artery disease and provided a theoretical basis for early prevention and clinical treatment.
Methods
This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Beijing Anzhen Hospital, Capital Medical University. Written informed consent was obtained from the participants.
Patients
Patients with TA involving the coronary artery were included in this study. The diagnosis standard of aortitis was according to the 1990 American Rheumatology Association (ACR) diagnosis standard of aortitis (9). The assessment of the patients’ arteritis activity was according to the NIH standard (10). According to the patients’ condition of coronary artery involvement, they were divided into two groups: group A: TA involved coronary artery disease [at least one coronary artery stenosis (≥50%)] and group B: TA did not involve the coronary artery. This study was approved by the ethics committee of our hospital and all patients signed informed consent.
Inclusion and exclusion criteria
Inclusion criteria: (I) patients who were diagnosed as TA; (II) patients with at least one coronary artery stenosis ≥50%; (III) age was older than 18-years-old; (IV) patients who had signed informed consent. Exclusion criteria: (I) patients with severe infections; (II) patients with severe liver or kidney dysfunction; (III) patients with severe coagulation dysfunction;(IV) patients whose data was incomplete; (V) patients with congenital heart diseases, such as coarctation of aorta, fibromuscular dysplasia, pulmonary heart disease, etc.; (VI) patients with systemic inflammatory diseases, such as rheumatoid arthritis, systemic lupus erythematosus or other primary and secondary vascular diseases; (VII) patients with malignant tumor, hematological system disease, and other acute and chronic infections.
The main observational index
In this study, we recorded the patients’ age, gender, height, weight, body mass index (BMI), smoking history, diabetes history, hypertension history, history of dyslipidemia, and family history of cardiovascular disease. Besides, blood pressure, heart rate, heart rate, vascular murmur was also recorded.
The patients’ blood index: venous blood was drawn in the early morning of the next day after hospitalization (fasting ≥8 hours), blood routine was detected by an automatic blood analyzer, ESR was detected by srs100/II, hsCRP was detected by immunoturbidimetry (Beckman AU5400), and hsCRP was detected by Beckman AU5400. The biochemical indexes were analyzed by a biochemical analyzer: serum creatinine (SCR), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride (TG). The evaluation index of coronary artery disease was completed by coronary angiography or CTA. All patients received color Doppler echocardiography and left ventricular ejection fraction (LVEF) and the results were recorded.
Statistical analysis
We used the software program SPSS 20.0 (IBM, Chicago, USA) to conduct the statistical analysis. Continuous variables were expressed as mean± SD and discontinuous variables were expressed as a percentage (%). For two comparisons, each value was compared by a t-test when each datum conformed to a normal distribution while the non-normally distributed continuous data were compared using non-parametric tests. The counting data were tested by a chi-square test. The risk factors of TA involvement in coronary artery disease were analyzed by a multivariate logistic regression model and a value of P<0.05 was considered statistically significant.
Results
General data
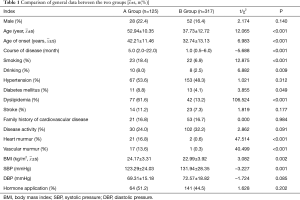
A total of 442 TA patients were included in this study. The average age was 41.92±13.99 years. The patients included 80 males (18.1%) and 362 females (81.9%). The ratio of men to women was approximately 1:4.5. Compared with group B, group A was older (52.54±11.17 vs. 37.73±12.72, P<0.001), had a later onset (42.21±11.46 vs. 32.74±13.13, P<0.001),longer (5.0 vs. 1.0, P<0.001), had a larger BMI (24.17±3.31 vs. 22.99±3.92, P=0.002), and had a higher proportion of smoking, drinking, diabetes, and dyslipidemia. The prevalence rate was higher and the difference between the two groups was statistically significant (all P<0.05); the proportion of heart murmur and vascular murmur was more evident in the patients’ physical signs and the difference between the two groups was statistically significant (P<0.001); there was no statistical significance between the two groups in gender, family history of cardiovascular disease, disease activity, and the proportion of applied hormone (P>0.05) (Table 1).
hsCPR, ESR, biochemical indexes, and cardiac function
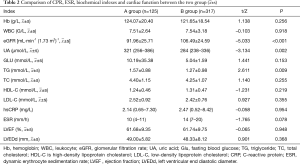
Compared with group B, the eGFR level in group A was significantly lower (91.96±25.71 vs. 106.49±24.59, P<0.001) and the UA and TG levels were significantly higher (P<0.05), while LDL-C, hsCRP, ESR, and left ventricular ejection fraction (LVEF) had no significant difference between the two groups (P>0.05) (Table 2).
Full table
The multivariate analysis of the risk factors of TA involving the coronary artery
A multiple logistic regression analysis was carried out with the patients’ sex, age, age of onset(first onset of clinical symptoms), course of TA, BMI, smoking history, drinking history, hypertension, dyslipidemia, diabetes mellitus, and disease activity as the independent variables. The results showed that age of onset (OR =1.143, 95% CI: 1.007–1.298, P=0.039), course of TA (OR =1.165, 95% CI: 1.025–1.324, P=0.020), and BMI (OR =1.100, 95% CI: 1.021–1.185, P=0.013) were the risk factors of TA involving the coronary artery (Table 3).

Full table
Discussion
Cases of TA are found worldwide but it is more common in Asia and the Middle East and the disease is rare. Its prevalence is estimated to be 1–2/1 million in Japan and 2.2/1 million in Corvette. Epidemiological studies from Europe show that the incidence varies from 0.4–1.3/1 million. A recent National Hospital incidence study from Poland showed that the incidence of TA was estimated to be 0.92/1 million per year. TA mostly occurs in young women and the proportion of men and women in different groups around the world is different, mostly 1 (5-11). The age of onset is mainly 5–40-years-old, the peak is 20–30-years-old, and there is a main peak between 15–19-years-old in female patients (11,12). A Japanese study evaluated the clinical characteristics of newly diagnosed TA patients and the disease’ s association with age and gender. The results showed that the male to female ratio of newly diagnosed TA patients was 1:5 and the median age of onset in male patients was significantly higher than that in female patients; 43.5-years-old and 34-years-old, respectively. Approximately 85% of the patients are aortic or including its main branches. Young female patients tend to have localized lesions while male patients often have extensive aortic lesions or aneurysms with more complications. However, local abdominal lesions are relatively common in male patients over 40-years-old (13,14). The incidence of TA involvement in the coronary artery was reported in 10–30% of cases (8,15,16). In this study, 442 patients with TA were included, the ratio of male to female was 1:4.5, and the average age of onset was (35.35±13.37) years. The average age of onset was (34.70±13.66) years for women and (38.37±11.52) years for men. The incidence of coronary artery involvement was approximately 28.2%, which was similar to the abovementioned epidemiological data.
The prognosis of TA is related to the involvement of the patients’ heart, brain, and other important organs, as well as the location of the involved vessels. A survey on the mortality and causes of death of systemic connective tissue diseases and primary systemic vasculitis from Norway showed that the estimated standard mortality of TA was the highest in primary systemic vasculitis and the main causes of death were cardiovascular disease, tumor, chronic respiratory disease, and infection (17). Coronary artery ischemia was also a recognized cause of death in these patients (18). Therefore, it is of great significance to screen the risk factors of coronary artery involvement in TA patients and to take appropriate prevention and control measures to improve the prognosis of TA patients.
The expansion of chronic aortic inflammation to the opening and near segment of the coronary artery can cause the stenosis of the coronary artery lumen and the inflammation leads to the destruction of the elastic fibers of the intima and media of the artery, the weakness of the wall of the artery, and the expansion of the aneurysm (19), which can cause myocardial ischemia. But inflammation is not the only mechanism of TA patients involving coronary artery disease. Previous studies have shown that vascular calcification in TA is not only related to inflammation but also related to age and the use of glucocorticoids (7). Glucocorticoid therapy has a complex relationship with the development of atherosclerosis. Abnormal lipid metabolism, abnormal glucose metabolism, and high blood pressure are significant cardiovascular side effects of glucocorticoid therapy. The effects of glucocorticoid therapy on the lipid spectrum mainly include VLDL, TG, and LDL-C, while HDL-C can be increased or decreased, while the increase of serum cholesterol (mainly LDL-C) is positively correlated with the occurrence of atherosclerosis (20). Therefore, glucocorticoid therapy may be one of the factors that affect coronary artery disease in TA patients (21).
Alarcón García (8) found that LP (a) [Lp (a)] was significantly increased in TA patients and LP (a) had the effect of atherosclerosis, which was a risk factor for cardiovascular disease. Alibaz-Oner et al. (22) used ultrasound to detect flow-mediated dilation (FMD) of the brachial artery to evaluate endothelial dysfunction and to detect intima-media thickness (IMT) of the carotid artery to understand the change of the atherosclerotic structure. The results showed that the FMD in TA patients decreased significantly and CIMT increased, indicating the inflammatory state and blood vessels of TA patients. Inflammation can lead to obvious endothelial dysfunction and can increase the occurrence of atherosclerosis. TA involves the mechanism of coronary artery disease. TA is mainly characterized as an inflammatory disease related to heredity and autoimmunity. The cellular immunity mediated by CD4+ T and CD8+ T cells can promote the formation of granuloma, activate the activity of matrix metalloproteinases and other proteases, promote the release of inflammatory factors such as IFN-α, TNF-α,IL-6, IL-8, and IL-18, and lead to the formation of chronic inflammation and fibrosis of the tube wall (23,24), resulting in the stenosis and occlusion of the lumen. The endothelium also promotes inflammatory changes and increases the permeability of dysfunction.
Low-density lipoprotein cholesterol (LDL-C) in the blood enters the intima and accumulates under the intima, which is the key initial step of atherosclerosis (25). Ox-LDL is an important proinflammatory factor, which can promote the release of MCP-1, TNF-α, IL-8, and other inflammatory mediators from endothelial cells, make monocytes adhere firmly and enter the vascular endothelium, and also stimulate the proliferation of vascular smooth muscle cells, thus, promoting the occurrence of atherosclerosis (26)]. Seyahi et al. (27) found that the incidence of carotid atherosclerotic plaques in TA patients was higher than that in healthy people of the same age and gender group. Plaques were mostly located in the lesions of the vessel wall of primary arteritis and age and high cholesterol level were significantly related to the occurrence of plaques in TA patients. Further analysis showed that age was a risk factor for the occurrence of atherosclerotic plaques in TA patients. It is suggested that the changes of turbulent flow and shear stress in stenosis and the pro-inflammatory changes of endothelium caused by inflammation damage of arterial wall, arterial function or anatomical structure are all related to the development of atherosclerosis in TA patients (28). Soto believes that TA patients with coronary artery disease have a higher prevalence, an older age, and a longer course of disease than in patients without coronary artery disease (29).
Renal dysfunction can activate the Renin-angiotensin-aldosterone system and oxidative stress, increase the synthesis of endothelin and inflammatory factors, increase the production of oxygen free radicals in cells, and often combine with a variety of risk factors such as old age, obesity, smoking history, hypertension, diabetes, dyslipidemia, hyperuricemia, etc., which lead to endothelial dysfunction promoting the atherosclerosis process (30). The results of the multiple logistic regression analysis in this study showed that the age of TA onset, the course of TA, and BMI were independent risk factors of TA involvement in the coronary artery but were not related to the disease activity. It is suggested that long-term chronic vasculitis may lead to coronary artery stenosis in TA patients and autopsy pathological studies show that the inflammatory activity in TA patients is extensive but not active in the clinical manifestations, suggesting that there are some limitations in the current clinical methods in determining the disease activity and the activity is often underestimated (31), while in the clinical disease inactive stage, the vascular disease may still progress (32). The results of this study suggest that the traditional risk factors such as age, dyslipidemia, smoking, etc. are closely related to TA involvement in coronary artery disease. These results also showed that the later the onset age, the more susceptible patients are in developing coronary artery involvement. This further suggests that the older the patient’s age, on the one hand, combined with various risk factors, various physical and chemical factors easily lead to coronary artery endothelial damage, while on the other hand, the inflammatory damage of TA itself, both of which jointly promote the progress of coronary lesions. These findings are similar to those of Soto and other research reports.
Limitations. There were several limitations to this study. Firstly, this trial was only a retrospective study and not a randomized controlled trial. Secondly, this study was only a single-center trial and the sample size was limited. Thirdly, the clinical follow-up was short, and it was necessary to observe the clinical long-term prognosis. In the future, we will try to carry out another multi-center, prospective research with a large sample size and an in-depth study of the risk factors of TA coronary artery involvement and explore the relevant mechanisms to provide more theoretical bases for better prevention and treatment of TA coronary artery disease.
In summary, this study showed that the occurrence and development of TA patients' involvement in coronary artery disease are multifactorial. Besides inflammation, traditional risk factors also play an important role, which may promote the occurrence of atherosclerosis in patients with a systemic inflammatory disease or the interaction between TA inflammation itself and traditional risk factors of cardiovascular disease may promote the progress of coronary artery disease. It is suggested that we be alert in recognizing the involvement of coronary artery, screen coronary artery lesions on time, and pay close attention to the comprehensive control of the traditional risk factors while controlling inflammation, which is very important in improving the prognosis of TA in patients.
Acknowledgments
Funding: National Natural Science Foundation of China, No. 81974548.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-267). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted with approval from the Ethics Committee of Beijing Anzhen Hospital, Capital Medical University (IRB number: XXXXXXXXXXX). Written informed consent was obtained from the participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mirault T, Messas E. Takayasu arteritis. Rev Med Interne 2016;37:223-9. [Crossref] [PubMed]
- Numano F. The story of Takayasu arteritis. Rheumatology 2002;41:103-6. [Crossref] [PubMed]
- Mahajan N, Dhawan V, Mahmood S, et al. Extracellular Matrix Remodeling in Takayasu\"s Arteritis: Role of Matrix Metalloproteinases and Adventitial Inflammation. Arch Med Res 2012;43:406-10. [Crossref] [PubMed]
- Arnaud L, Haroche J, Mathian A, et al. Pathogenesis of Takayasu's arteritis: A 2011 update. Autoimmun Rev 2011;11:61-7. [Crossref] [PubMed]
- Hoffman GS, Merkel PA, Brasington RD, et al. Anti–tumor necrosis factor therapy in patients with difficult to treat Takayasu arteritis. Arthritis Rheum 2004;50:2296-304. [Crossref] [PubMed]
- Kang EJ, Kim SM, Choe YH, et al. Takayasu arteritis: assessment of coronary arterial abnormalities with 128-section dual-source CT angiography of the coronary arteries and aorta. Radiology 2014;270:74-81. [Crossref] [PubMed]
- Banerjee S, Bagheri M, Sandfort V, et al. Vascular calcification inpatients with large-vessel vasculitis compared to patients with hyperlipidemia. Semin Arthritis Rheum 2019;48:1068-73. [Crossref] [PubMed]
- Alarcón García JC, Rodríguez Suárez S, Muñiz Grijalvo O, et al. Vascular lesions in patient with Takayasu arteritis and massive elevated lipoprotein(a) levels. Residual involvement or premature aterosclerosis? Clin Investig Arterioscler 2017;29:98-102. [Crossref] [PubMed]
- Arend WP, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum 1990;33:1129-34. [Crossref] [PubMed]
- Kerr GS, Hallahan CW, Giordano J, et al. Takayasu arteritis. Ann Intern Med 1994;120:919-29. [Crossref] [PubMed]
- Watanabe Y, Miyata T, Tanemoto K. Childhood-onset Takayasu arteritis: an update. Circulation 2015;132:1701-9. [Crossref] [PubMed]
- Liu H, Sun L, Upadhyaya RS, et al. Case report: Takayasu arteritis in a 3-month-old Chinese girl. Medicine (Baltimore) 2018;97:e12637. [Crossref] [PubMed]
- Mohan S, Poff S, Torok KS. Coronary artery involvement in pediatric Takayasu's arteritis: Case report and literature review. Pediatr Rheumatol Online J 2013;11:4. [Crossref] [PubMed]
- Li J, Li H, Sun F, et al. Clinical Characteristics of Heart Involvement in Chinese Patients with Takayasu Arteritis. J Rheumatol 2017;44:1867-74. [Crossref] [PubMed]
- Rav-Acha M, Plot L, Peled N, et al. Coronary involvement in Takayasu’s Arteritis. Autoimmun Rev 2007;6:566-71. [Crossref] [PubMed]
- Khor CG, Tan BE, Kan SL, et al. Takayasu Arteritis in Major Rheumatology Centers in Malaysia. J Clin Rheumatol 2016;22:194-7. [Crossref] [PubMed]
- Garen T, Lerang K, Hoffmann-Vold AM, et al. Mortality and causes of death across the systemic connective tissue diseases and the primary systemic vasculitides. Rheumatology (Oxford) 2019;58:313-20. [Crossref] [PubMed]
- Park YB, Hong SK, Choi KJ, et al. Takayasu arteritis in Korea: clinical and angiographic features. Heart Vessels Suppl 1992;7:55-9. [Crossref] [PubMed]
- Abou Sherif S, Ozden Tok O, Taşköylü Ö, et al. Coronary Artery Aneurysms: A Review of the Epidemiology, Pathophysiology, Diagnosis, and Treatment. Front Cardiovasc Med 2017;4:24. [Crossref] [PubMed]
- Skålén K, Gustafsson M, Rydberg EK, et al. Subendothelia retention of atherogenic lipoproteins in early atherosclerosis. Nature 2002;417:750-4. [Crossref] [PubMed]
- Ross IL, Marais AD. The influence of glucocorticoids on lipid and lipoprotein metabolism and atherosclerosis. S Afr Med J 2014;104:671-4. [Crossref] [PubMed]
- Alibaz-Oner F, Yurdakul S, Aytekin S, et al. Impaired endothelial function in patients with Takayasu's arteritis. Acta Cardiol 2014;69:45-9. [Crossref] [PubMed]
- Noris M. Pathogenesis of Takayasu's arteritis. J Nephrol 2001;14:506-13. [PubMed]
- Tanaka A, Tearney GJ, Bouma BE. Challenges on the frontier of intra coronary imaging: atherosclerotic plaque macrophage measurement by optical coherence tomography. J Biomed Opt 2010;15:011104. [Crossref] [PubMed]
- Mitra S, Deshmukh A, Sachdeva R, et al. Oxidized low-density lipoprotein and atherosclerosis implications in antioxidant therapy. Am J Med Sci 2011;342:135-42. [Crossref] [PubMed]
- Zernecke A, Weber C. Chemokines in atherosclerosis: proceedings resumed. Arterioscler Thromb Vasc Biol 2014;34:742-50. [Crossref] [PubMed]
- Seyahi E, Ugurlu S, Cumali R, et al. Atherosclerosis in Takayasu arteritis. Ann Rheum Dis 2006;65:1202-7. [Crossref] [PubMed]
- Mason JC, Libby P. Cardiovascular disease in patients with chronic inflammation: mechanisms underlying premature cardiovascular events in rheumatologic conditions. Eur Heart J 2015;36:482-9c. [Crossref] [PubMed]
- Soto ME, Meléndez-Ramírez G, Kimura-Hayama E, et al. Coronary CT angiography in Takayasu arteritis. JACC Cardiovasc Imaging 2011;4:958-66. [Crossref] [PubMed]
- Amann K, Wanner C, Ritz E. Cross-talk between the kidney and the cardiovascular system. J Am Soc Nephrol 2006;17:2112-9. [Crossref] [PubMed]
- Watson L, Brogan P, Peart I, et al. Diagnosis and assessment of disease activity in takayasu arteritis: a childhood case illustrating the challenge. Case Rep Rheumatol 2014;2014:603171. [Crossref] [PubMed]
- Soeiro Ade M, Pinto AL, Henares BB, et al. Takayasu arteritis: stenosis after bare-metal and drug-eluting stent implantation. Arq Bras Cardiol 2013;100:e8-11. [PubMed]