
Clinical usefulness of bronchoalveolar lavage in patients with interstitial lung diseases: a pilot study
Introduction
Interstitial lung diseases (ILDs), also known as diffuse lung parenchymal diseases (DLPD), are a heterogeneous group of more than 100 entities that may share some common clinical, radiographic, physiologic and pathologic manifestations, however have differences in their underlying etiology, molecular pathophysiology and prognosis. The current classification scheme consists of disorders of known causes, [e.g., connective tissue disease (CTD)], disorders of unknown causes [also termed as idiopathic interstitial pneumonias (IIPs)], granulomatous lung diseases (e.g., sarcoidosis) and other miscellaneous ILDs (1). Given their etiological complexities, the diagnosis and management of ILD are a challenge to physicians. Multidisciplinary approach is recommended as the preferred process to establish a diagnosis of IIPs, but its role in management of other forms of ILDs remains elusive (2).
Bronchoalveolar lavage (BAL) is widely accepted as a simple and relatively safe diagnostic procedure that allows sampling cellular and acellular components of the lower respiratory tract. Analysis of the retrieved BAL fluid (BALF) can lead to a diagnosis of a variety of lung disorders (3,4). BALF analysis was considered characteristic in selected ILD, such as strengthening the diagnosis in patients with sarcoidosis in the absence of lung biopsy and even replacing lung biopsy in patients with pulmonary alveolar proteinosis (PAP) (4-7). Nevertheless, in other settings, BALF analysis is not specific and its role in achieving a diagnosis or directing therapy has not yet been well established (4,7,8).
In this study, we liked to explore the clinical usefulness of BAL in aiding a diagnosis of patients with ILD in different clinical setting.
Methods
Patient selection
This study was approved by the Institute Review Board of Taipei Veterans General Hospital (VGHTPE No. 2010-09-019IC). Consecutive 184 patients older than 16 years with radiographic evidence of ILDs by high-resolution computed tomography (HRCT) of the chest and serial chest radiograms who were eligible for diagnostic BAL to establish a diagnosis of ILD at our institute from January 2017 to December 2017 were included. Radiographic evidence of ILDs was defined as (I) linear and reticular shadowing; (II) small or miliary nodules; (III) reticulonodular opacities; (IV) honeycombing lesions; (V) interlobar septal thickening and (VI) ground-glass opacities (GGO) with or without patch infiltrations or consolidation. All patients signed an informed consent before entering the study. There were 37 outpatients (OPD group), 86 patients admitted to general ward (GW group) and 61 patients admitted to intensive care unit (ICU group). Exclusion criteria were as follows: undergoing bronchial washing alone, localized lesions on imaging studies, without providing the informed consent, and inadequate specimens as evaluated independently by a qualified cytologist. The process to identify study population is shown in Figure 1.
BAL assessment and specimen sampling
The BAL protocol including the pre-procedure preparation and BAL procedure was done in accordance the official American Thoracic Society (ATS) clinical practice guideline: the clinical utility of BAL cellular analysis in ILD (9). In brief, the fiberoptic bronchoscope (Model FB 20 or P20; Olympus, Tokyo, Japan) was wedged in the orifice of a lobar or segmental bronchus of the right middle lobe or lingular division or other appropriate location based on the findings of chest images. Diagnostic BAL was done using three aliquots of a 50-mL sterile isotonic sodium chloride. The fluid was aspirated and pooled into a siliconized container and kept on ice during transport (4,10). Part of the retrieved BALF was subjected to Papanicolaou and Liu’s staining routinely. Some slides were stored for subsequent special staining if clinically indicated (11,12).
BALF cell analysis
In brief, BALF for cell analysis was filtered through two-layer sterile gauzes to remove mucus, and then cellular materials were sedimented by centrifugation (2,500 rpm for10 min at 4 °C) and the supernatant was stored at −70 °C for cytokine analysis later. The pellet was re-suspended in 1 mL phosphate buffered saline (PBS) for quantitative cell count. The total cell count was measured by a hemocytometer (Hausser Scientific, Horsham, PA, USA) and cell differentials were calculated by cytological smears with Liu’s staining for counting at least 300 cells. The lymphocytes >15–18%, neutrophils >3–5% (>5% in smoker or exposure to heavy air pollution) and eosinophils >1% were defined as abnormal cellular patterns (9).
Diagnosis of the causes of ILD
A diagnosis of Pneumocystis jirovecii pneumonitis (PJP), cytomegalovirus (CMV), pneumonia was made when pathogens were identified in the cytological smears of BALF. The diagnosis of bacterial infection is made when bacterial culture yielded bacterial colony more than 104. A diagnosis of idiopathic or autoimmune pulmonary alveolar proteinosis (iPAP) was made based on the presence of characteristic findings highly suggestive of iPAP in cytological smears of BALF (11). The diagnosis of drug induced lung injury (DILI) is based on the following criteria: (I) treatment with the drug; (II) newly developed pulmonary lesions presenting as ILD on the imaging studies after the use of the drug; (III) other causes of lung diseases were extensively excluded clinically; (IV) obvious clinical and radiological improvement after discontinuation of the drug and good response of lung lesions to steroid treatment. The drugs might cause DILI including tyrosine kinase inhibitor against the human epidermal growth receptor, sirolimus (immunosuppressant for solid organ transplantation), amiodarone and statin in this study. The hypersensitivity pneumonia was defined by an exposure history and consistent cellular pattern presenting as lymphocytosis without increased mast cells and eosinophils, compatible thoracic imaging and good response of lung lesions to steroid treatment. The diagnosis of sarcoidosis was defined as tissue proved granulomatous inflammation of mediastinal and/or lymph node biopsy of in addition to consistent thoracic imaging, marked lymphocytosis of BALF and good response of thoracic lesions to steroid treatment. The diagnosis of radiation pneumonitis (RP) was diagnosed based on previous history of radiotherapy, the time interval between the end of radiotherapy and the occurrence of newly developed pulmonary lesion that is not unusual for making a diagnosis of RP, the findings on HRCT of the chest are not unusual for RP, the BAL cellular pattern is not unusual for the results of RP, and good response of steroid treatment. Pulmonary edema is diagnosed by the presence of iron laden macrophages shown on cytological smear, comet rail sign shown on chest sonography after BAL with subsequent echocardiogram. Accordingly, the diagnosis of ILD is not based on the BAL study only and in combination with clinical features, radiologic findings, lab tests, treatment response and even transbronchial lung biopsy (TBLB) or lung biopsy. In general, our final diagnosis was made by two senior chest physicians and the clinical usefulness is also based on the consensus of the two senior chest physicians.
Diagnostic yield of BAL
Diagnostic yield was recorded when a diagnosis of the cause of underlying ILD was altered and affecting the management based on the results of BAL mainly. These included a new diagnosis was made by BAL including cytological smears and microbiological exam, and molecular tests [polymerase chain reaction (PCR)] of BALF, pertinent clinical features, thoracic images, other clinical tests and clinical extensive exclusion. In addition, TBLB and lung biopsy guided with chest ultrasonography were done in certain cases. Adding a new diagnosis (mixed entities) of the causes of ILD with affecting management was made by BAL as described above. Iron staining was done in the following condition: in immunocompromised hosts, in patients with bloody BALF and in some patients ordered by the physicians.
Data collection & statistical analysis
Demographic, clinical and laboratory data were collected, with emphasis on the diseases associated with ILD, past and current medications, use of antimicrobials within previous weeks before BAL done, and microbiological studies of blood, sputum and/or low respiratory secretions, pleural effusion or other body fluids. All inpatients were followed up until discharge or death. BALF cytology was interpreted by two experienced and qualified cytologists routinely. Etiology was established based on BALF cytology, other diagnostic procedures, clinical course, treatment response and outcome. The infectious etiology was determined using strict criteria (11). At least two senior investigators reviewed all clinical information available and a consensus of final diagnosis was achieved in every case. Statistical analysis was performed using chi-square test and/or Fisher’s exact test, when appropriate. A P value <0.05 was considered statistically significant.
Results
Patient characteristics
Of the 184 patients included into this study, there were 37 outpatients (OPD group), 86 patients admitted to general ward (GW group) and 61 patients admitted to intensive care unit (ICU group). The gender ratio (male/female) was 16/21 in OPD group, 35/51 in GW group and 34/27 in ICU group, respectively. The mean age of the patients was 63.1±15.8 years (range, 25–89 years) for OPD group, 63.2±18.1 years (range, 21–89 years) for GW group and 59.7±17.0 years (range, 17–80 years) for ICU group, respectively. There was no significant difference in the age and gender among the three groups. In light of co-existing major diseases, 57 patients had CTDs, 24 patients had active hematologic or solid organ malignancies and 17 patients underwent hematopoietic stem cell or solid organ transplantation.
The use of antibiotics before BAL
Antibiotics were not used in the patients of OPD group. On the contrary, antibiotics were all used in the admitted patients because of pyogenic pneumonia could not be excluded in very patient of GW and ICU groups. The most commonly used antibiotics and anti-fungal agents included tazocin, levofloxacin, and fluconazole, which were used for about 3–7 days in most patients before BAL.
Diagnostic yield of BAL
Overall, diagnostic yields of BAL among OPD group, GW group and ICU group were 83.6%, 74.4% and 78.4%, respectively (Table 1). Our data indicated that there was significant difference in etiologies of ILD among the three groups (P<0.001, Table 1). Infectious etiologies occurred more frequently in ICU group than another two groups. Mixed entities were seen in inpatients (both GW and ICU groups) alone. BAL failed to disclose the underlying causes of ILD in 16.5–25.7% of the patients. When compared in pair, the distribution of etiologies of ILD was significantly different for ICU group versus GW group (P=0.004) and for ICU group versus OPD group (P<0.001).
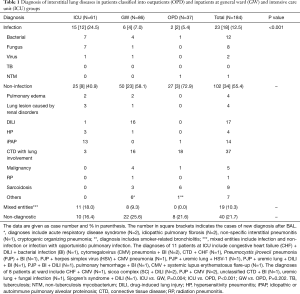
Full table
Revision of ILD diagnosis after BAL
With a diagnostic result, BAL might provide evidence to support the clinical impression before the procedure, or even lead to an alternative working diagnosis that further guided additional tests or altered the treatment. Revision of ILD diagnosis (including new diagnosis and mixed etiologies) after BAL among OPD group, GW group and ICU group are summarized in Table 2. Diagnosis was revised 21.6% in OPD group, 69.7% in GW group and 60.6% in ICU group patients, respectively. Revision of diagnosis was much more commonly noted in inpatients, either in GW group or ICU group as compared with outpatients (P<0.001).

Full table
Etiologies of ILD in patients with co-existing major diseases
Table 3 shows the distribution of etiologies of ILD among patients with CTD (N=57), malignancies (N=24) and organ transplantation (N=17). There was significant difference in the etiologies of ILD in the patients with these three co-existing major diseases (P=0.009). The overall diagnostic yield was significantly lower in patients with malignancy, with non-diagnostic rate up to 37.5%, compared with other two groups (CTD, 14.0%; organ transplantation, 23.5%) and the whole study population (21.7%). Infectious etiologies accounted for a very small portion (3.5%) of ILDs in patients with CTD, in contrast to other two groups (malignancies, 16.7%; organ transplantation, 23.5%). Non-infectious etiologies were responsible for a non-negligible part of ILD among the three patient groups (29.2–70.2%). The most common non-infectious cause for patients with CTD was pulmonary involvement of their underlying diseases (37/40). The two major non-infectious causes in patients with organ transplantation were DILI and pulmonary edema secondary to renal diseases (Table 3).
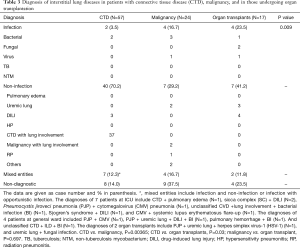
Full table
Discussion
In patients with radiographic evidence of ILD, BAL is a safe, minimally invasive procedure across a wide range of clinical settings and can provide useful information about etiologies. We found overall yields among OPD group, GW group and ICU group were 78.4%, 74.3% and 83.5%, respectively. To our knowledge, this was the first study to systematically evaluate the diagnostic utility of BAL in such heterogeneous, unselected patients. Most published studies focused on the role of BAL in patients with confirmed, selective ILDs or in a work-up of pulmonary infiltrates in immunocompromised hosts. In a recently published retrospective observational study, Efared et al. looked for BALF cytological analysis in a patient group largely composed of sarcoidosis and IIPs and concluded that it had limited value to discriminate between specific forms of ILD (13). In another recent study, the authors also analyzed alveolar cellular profile and reported that BALF cytological analysis confirmed the diagnosis in 60% of patients with pneumoconiosis, 45% of patients with hypersensitivity pneumonitis and 35% of patients with sarcoidosis, as well as made it possible to exclude idiopathic pulmonary fibrosis in 12% of patients (13). Infections or other non-infectious entities such as DILI or pulmonary hemorrhage were not mentioned in these two studies (12,13). After all, the participants in our study were much more heterogeneous in composition than others. Patients with “typical” ILD or DPLD, such as sarcoidosis and IIPs, consisted of only a very small portion (Table 1), but our approach was more resembling real-world daily practice when physicians facing a patient with radiographic evidence of ILD. BALF cellular analysis alone is insufficient to diagnose the certain types of ILD, but combined with appropriate microbiological studies of BALF, these findings may support a specific diagnosis when considered in the context of the clinical presentations and systemic disease (7,14). Moreover, the diagnosis of ILD was revised after BAL in significant portions among the three groups (21.6% in OPD group; 69.7% in GW group; 60.6% in ICU group; Table 2). It is worth mentioning that despite a low diagnostic yield in ICU patients, the possibility of diagnosis revision was relatively high, and the reverse was found in outpatients.
Another well-established role of BAL is evaluation of immunocompromised patients with pulmonary infiltrates or suspected pneumonia. Joos et al. performed BAL in 1,066 immunocompromised patients with suspected pneumonia, largely composed of HIV-positive patients and those with hematological malignancies, in a 12-year period and reported the overall diagnostic yield for pulmonary infections was as high as 85.7% (15). Although conventional cytology was performed as well, non-infectious etiologies were not documented. In another study with similar design, Vélez et al. found an overall yield of 51.6% but up to 75.9% for diagnosing infectious etiologies (12). The list of non-infectious etiologies was short, including pulmonary fibrosis, malignancy infiltration, pulmonary edema and pulmonary hypertension (12). Both studies confirmed that BAL had a high diagnostic yield in immunocompromised patients with suspected pneumonia. However, based on our clinical practice we question that impaired immunity of non-acquired immunodeficient syndrome (AIDS) immunocompromised patients may be quite different. In addition, the causes of ILD may be due to underlying diseases involve with lungs or complication of the therapy for controlling the underlying diseases. Our study population also comprised of a significant number of immunocompromised hosts (CTD, N=57; malignancy, N=24; organ transplantation, N=17 patients; Table 3). Infectious etiologies accounts for a relatively minor part in final diagnosis: 5.4% in OPD group, 6.9% in GW group and 24.5% in ICU group, respectively (Table 1). For the immunocompromised patients, infectious etiologies still accounted for a significantly smaller percentage in final diagnosis: 3.5% in CTD patients, 16.7% in malignancy patients and 23.5% in organ transplantation patients (Table 3), a finding that was quite different from previous studies (9,11). The discrepancies of the results between our and previous studies (9,11) might be partly explained by that HIV-positive patients were not included in the present study. In addition, the admitted patients (GW group and ICU group) in our study might be treated with empiric antibiotics for a period before BAL was performed. As a result, certain patients with ILD caused by infectious causes might be excluded and falsely reduced the role of infectious cause in ILD patients. The above explanation was supported by the almost all infectious etiologies identified by BAL in our study were resistant organisms, virus or fungi. Of note, infectious etiologies were still more common in ICU patients than in other groups (Table 1). The overall diagnostic yield was 84.0% in CTD patients, 63.5% in malignancy patients and 76.5% in organ transplantation patients despite a lower percentage of infectious etiologies in our study. This could be explained that we identified a greater number of non-infectious etiologies that were rarely reported in other studies (9,12), such as DILI and RP. It is worth noting that a higher proportion of patients (N=40) with CTD to be diagnosed to have ILD secondary to their underlying disorders (Table 3).
CTD, also called collagen-vascular disease, represents a heterogeneously group of immunologically mediated inflammatory disorders with a variety of affected organs. The overall incidence of ILD across the spectrum of CTD is estimated to be 15%, but the data vary widely in individual diseases (15). CTD has been estimated to be an uncommon cause of ILD (16). In a recently published French cohort study including 1,170 patients, CTD accounted for 16% of cases with ILD, but contribute to 56% cases with ILD of known causes (17). In our study, 37 patients were diagnosed to have CTD-ILD (37/184, 20.1%), a number which was slightly higher than those reported in the literature and accounted for most of non-infectious etiologies in patients with CTD (37/40, 92.5%, Table 3). Most of them had been labelled as CTD before being included into this study and were referred from the rheumatologists. A small portion of them was found to have certain forms of CTD after BAL and additional assessment.
Definite diagnosis of ILD still could not be made in a significant number of patients after BAL: 8 (21.6%) in OPD group, 22 (25.6%) in GW group and 10 (16.4%) in ICU group. In terms of co-existing diseases, non-diagnostic BAL was most frequently seen in patients with malignancy (9/24, 37.5%), followed by patients with organ transplantation (4/17, 23.5%) and CTD (8/57, 14.0%). Because surgical lung biopsy or autopsy was not performed, it was impossible to elucidate the causes of ILD in these patients with non-diagnostic BAL results. BAL as a diagnostic modality has several inherent limitations, such as nonspecific cytological presentations for many ILDs, sampling errors or low sensitivity in detecting abnormalities in diseases that does not involve the airway (4,7-9,18). We believed that at least some of these patients with non-diagnostic BAL likely suffered from drug-induced lung injury, but available clinical information could not fulfill the established criteria suggested by current literature (19). Moreover, mixed etiologies were found in inpatients: 8 (9.3%) in GW group and 11 (18.0%) in ICU group, but none in OPD group (Table 1). This finding might suggest a higher complexity in medical problems in inpatients than in outpatients and highlight the importance to consider more than one disease process when an admitted patient presented with radiographic evidence of ILD.
Our study had several important limitations. First, selection bias could be an issue because all participants subjected to BAL were selected by the consultant chest physicians who were major in ILD based on their clinical judgement. The timing of BAL being performed, a potential factor of diagnostic yield, depended on both recognition of suspected ILD patterns and referral to the specialists. BAL might not be done timely enough in some patients, but its impact on the yield was unable to be assessed in this study. Of note, some patients were not included in this study when their BAL was done by other chest physicians due to different techniques and protocol of BALF processing. Meanwhile, not all eligible patients with radiographic evidence of ILD underwent BAL due to preference and decision of patients and their physicians in charge. Our results might be more or less representative of daily practice in a single tertiary referral center and therefore the results of the present study should be extrapolating to other clinical settings with precautions. Second, the incidences of infections across all subgroups were likely underestimated. It was because not only many of our participants, especially inpatients, had been treated with empiric antimicrobials before BAL but also we did not include the most sensitive diagnostic assays, i.e., molecular testing, to rule out the presence of “atypical germs” and/or viruses that were difficult to be isolated using traditional methods. Moreover, surgical lung biopsy or imaging-guided transthoracic lung biopsy was not performed to correlate or confirm BAL findings. Though BAL cytology could suggest certain etiologies in the milieu of other relevant clinical information, interpretation might be inevitably arbitrary (4,7,8). Finally, the design of this study did not aim at parameters of patient outcomes, such as mortality, duration of hospital or ICU stay. Our study clearly showed the utility of BAL in patients with radiographic evidence of ILD, whether this high diagnostic yield could actually convert to or tailor treatment leading to improve outcomes warranted additional studies. However, our results may be of clinical relevance because epidermal growth factor receptor-tyrosine kinase inhibitors and anti-programmed death-a/programed death ligand 1 therapy are increasing used in non-small cell lung cancer and other malignancies (20-22), interstitial pneumonitis caused by these new anti-neoplasm therapy will be increased. Further prospective multi-center studies with larger populations are needed to elucidate the impact of BAL in patients with ILD caused by non-infectious causes.
In summary, BAL appears to have a good diagnostic yield in patients with radiographic evidence of ILD, regardless of clinical settings and co-existing major diseases. Our findings highlighted that non-infectious etiologies were found more commonly than infectious ones in this patient population. Mixed etiologies were almost never seen in outpatients, and BAL might change a diagnosis more frequently in inpatients than in outpatients. In CTD patients, the majority of ILD are pulmonary involvement of their underlying diseases. A non-diagnostic BAL result is most often obtained in patients with malignancy.
Acknowledgments
Funding: This study was supported from a grant of Taipei Veterans General Hospital, Taipei (V100C-060). The founding source were not involved the study design, data analysis and interpretation, and creation and submission of the manuscript.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-19-3659). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Institute Review Board of Taipei Veterans General hospital (VGHTPE No. 2010-09-019IC) and written informed consent of all patient was obtained before entering the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- American Thoracic Society. European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 2002;165:277-304. Erratum in: Am J Respir Crit Care Med 2002 Aug 1;166(3):426. [PubMed]
- Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. [Crossref] [PubMed]
- Reynolds HY. Use of bronchoalveolar lavage in humans-past necessity and future imperative. Lung 2000;178:271-93. [Crossref] [PubMed]
- Meyer KC. Bronchoalveolar lavage as a diagnostic tool. Semin Respir Crit Care Med 2007;28:546-60. [Crossref] [PubMed]
- Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis 1999;16:149-73. [PubMed]
- Borie R, Danel C, Debray MP, et al. Pulmonary alveolar proteinosis. Eur Respir Rev 2011;20:98-107. [Crossref] [PubMed]
- Costabel U, Guzman J. Bronchoalveolar lavage in interstitial lung disease. Curr Opin Pulm Med 2001;7:255-61. [Crossref] [PubMed]
- Meyer KC, Raghu G. Bronchoalveolar lavage for evaluation of interstitial lung disease: is it clinically useful. Eur Respir J 2011;38:761-9. [Crossref] [PubMed]
- Meyer KC, Raghu G, Baughman RP, et al. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med 2012;185:1004-14. [Crossref] [PubMed]
- Baughman RP. Technical aspects of bronchoalveolar lavage: recommendations for a standard procedure. Semin Respir Crit Care Med 2007;28:475-85. [Crossref] [PubMed]
- Chou CW, Lin FC, Tsai HC, et al. The importance of pro-inflammatory and anti-inflammatory cytokines in Pneumocystis jirovecii pneumonia. Med Mycol 2013;51:704-12. [Crossref] [PubMed]
- Vélez L, Correa LT, Maya MA, et al. Diagnostic accuracy of bronchoalveolar lavage samples in immunosuppressed patients with suspected pneumonia: analysis of a protocol. Respir Med 2007;101:2160-7. [Crossref] [PubMed]
- Efared B, Ebang-Atsame G, Rabiou S, et al. The diagnostic value of the bronchoalveolar lavage in interstitial lung diseases. J Negat Results Biomed 2017;16:4. [Crossref] [PubMed]
- Costa E, Silva M, Rolo R. The role of Bronchoalveolar lavage in Interstitial Lung Diseases. Rev Port Pneumol 2006;2017:360-2. [PubMed]
- Joos L, Chhajed PN, Wallner J, et al. Pulmonary infections diagnosed by BAL: a 12-year experience in 1066 immunocompromised patients. Respir Med 2007;101:93-7. [Crossref] [PubMed]
- Antoniou KM, Margaritopoulos G, Economidou F, et al. Pivotal clinical dilemmas in collagen vascular diseases associated with interstitial lung involvement. Eur Respir J 2009;33:882-96. [Crossref] [PubMed]
- Coultas DB, Zumwalt RE, Black WC, et al. The epidemiology of interstitial lung diseases. Am J Respir Crit Care Med 1994;150:967-72. [Crossref] [PubMed]
- Duchemann B, Annesi-Maesano I, Jacobe de Naurois C, et al. Prevalence and incidence of interstitial lung diseases in a multi-ethnic county of Greater Paris. Eur Respir J 2017;50:1602419. [Crossref] [PubMed]
- Biederer J, Schnabel A, Muhle C, et al. Correlation between HRCT findings, pulmonary function tests and bronchoalveolar lavage cytology in interstitial lung disease associated with rheumatoid arthritis. Eur Radiol 2004;14:272-80. [Crossref] [PubMed]
- Schwaiblmair M, Behr W, Haeckel T, et al. Drug induced interstitial lung disease. Open Respir Med J 2012;6:63-74. [Crossref] [PubMed]
- Oshima Y, Tanimoto T, Yuji K, et al. EGFR-TKI-associated interstitial pneumonitis in nivolumab-treated patients with non-small cell lung cancer. JAMA Oncol 2018;4:1112-5. [Crossref] [PubMed]
- Naidoo J, Wang X, Woo KM, et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol 2017;35:709-17. Erratum in: Errata. J Clin Oncol 2017;35:2590. [Crossref] [PubMed]



