Clinicopathological characteristics of solitary cavitary lung cancer: a case-control study
Introduction
Lung cancer is the most common malignant tumor in China and the world, and identified as the main cause of cancer-related death (1,2). It has been reported that r cavitary lesions was presented in 2% to 25% of lung cancer (3), with larger tumor tissue size, more squamous cell histology and poor prognosis (4-6). In recent years, with the continuous development of high-resolution computed tomography (HRCT) and other radiological technologies, more and more cavitary lung nodules smaller than 3 cm were found. At present, clinicopathological characteristics of cavitary lung cancer are mainly about squamous cell carcinoma based on previous published studies. However, cavitary adenocarcinoma becomes more dominantly with the increasing incidence of adenocarcinoma. As a result, the composition and structure of cavitary lung cancer have been significantly changed as adenocarcinoma presented more, raising the requirement of reevaluation of clinicopathological features cavitary lesions.
The objective of this retrospective, case-control study was to investigate the clinicopathological features of solitary cavitary lung cancer ≤3 cm. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-426).
Methods
This retrospective, case-control study was carried out between April 2014 and June 2018 in a single academic thoracic surgery center at the China-Japan Friendship hospital, Beijing, China.
Study population
Patients were selected from the hospital clinical database between April 2014 and June 2018 using several inclusion criteria: (I) at least one CT scan was performed in our hospital, and no puncture biopsy or anti-tumor therapy was performed before CT scan; (II) solitary nodule with maximum diameter ≤3 cm were shown in CT images; (III) lung cancer was diagnosed pathologically after surgery or puncture treatment in our hospital; (IV) complete clinicopathological data were available. Patients were excluded from the study cohort if one of the following criteria was met: history of previous lung operation, metastatic lung adenocarcinoma, multiple lung nodules. As a result, a total of 946 patients were included in this study. The upper limit of the normal value for carcinoembryonic antigen (CEA) was 5 ng/mL. The study was approved by our institutional ethics board of China-Japan Friendship Hospital (No. 2018-13-K08).
Review of CT images
CT images were independently evaluated by a radiologist with more than 10 years’ experience in thoracic imaging diagnosis and a thoracic surgeon with more than 10 years’ experience in diagnosis and treatment of thoracic disease. The clinicopathological characteristics of the patients were not informed in advance to avoid any potential bias. Length measurements were carried out by manual cursor method in the Picture Archiving and Communication System (PACS). All measurement data were measured on the cross section for three times and the average value was taken.
Evaluation contents include tumor location (peripheral or central), maximum tumor diameter, edge morphology changes and adjacent structural changes (notch, spiculation, pleural indentation, vascular convergence and air bronchogram), nodule density (nonsolid, partial solid and solid) and internal structure of cavitation (separations and blood vessels). If the patient underwent positron emission tomography-computed tomography (PET-CT) examination, the maximum standard uptake value (SUVmax) of the lesion was recorded, with the critical value of 2.5.
Cavitation was defined as the presence of an air-containing space within the primary tumor and which was not identifiable as an airway. Peripheral lesions were defined as existence of margin arising from subsegmental or other distal bronchi and bronchiole (7). Non-solid lesions refer to the increased density of lung tissues without covering the original vascular and bronchial shadow that foil the region (8). Solid lesions refer to nodules with soft tissue density that completely conceal the vascular and bronchial shadows.
Pathological characteristics
All patients’ postoperative or puncture pathology reports were reviewed, and histological type, tumor tissue size, visceral pleural invasion (VPI), lymphovascular invasion (LVI), lymph node metastasis and epidermal growth factor receptor (EGFR) gene mutation status were recorded. All patients were staged according to the eighth edition of tumor-node-metastasis classification proposed by IASLC Lung Cancer Staging Project (9). According to the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification method to further subdivided adenocarcinoma into adenocarcinoma in situ (AIS), microinvasive adenocarcinoma (MIA) and invasive adenocarcinoma. The invasive adenocarcinoma was further subdivide into one of the following five predominant patterns: lepidic, acinar, papillary, micropapillary and solid predominant pattern (10).
Statistical analysis
IBM SPSS Statistics 22.0 (IBM, Chicago, IL, USA) was used for statistical analysis. Continuous variables with normal distribution were presents as mean ± standard deviation (SD); non-normal variables were reported as median (interquartile range). For comparison of continuous variables between groups, t-test (homogeneity of variance) or t-test (heterogeneity of variance) was used for those conforming to normal distribution and Mann-Whitney U test was used for those failing to conform to normal distribution. Categorical variables were represented by number of cases and percentage. Pearson χ2 test, continuous calibration χ2 test and Fisher’s exact test were used to analyze categorical variables. Multivariable analysis was used to identify the risk factors of cavitation. Variables with a P value less than 0.05 from univariable analysis were identified and selected into multivariable analysis. Receiver operating characteristic (ROC) curve was used to determine optimal cut-off values for continuous variables. The area under the curve (AUC) was adopted to measure the diagnostic power of a test. Youden index was used to select the optimal sensitivity and specificity from the ROC curves. All tests were two-tailed and P<0.05 was considered statistically significant. Delete cases with missing data.
Results
Patients characteristics
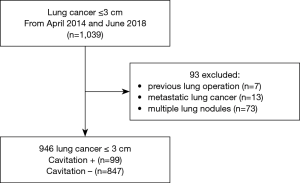
From April 2014 to June 2018, lung cancer ≤3 cm was confirmed in 1,039 patients.
Of these, 93 patients were excluded because of previous lung operation (n=7), metastatic lung cancer (n=13), multiple lung nodules (n=73). After exclusion, 946 patients (99 patients who were positive for cavitation and 847 patients who were negative for cavitation) remained (Figure 1). The mean age of our study population was (60±11) years and it included 406 men and 540 women. Two hundred and fifty patients (26.4%) had smoking history and 146 patients (15.4%) were CEA-positive; 44 patients underwent CT-guided puncture biopsy of chest lesions, and 902 patients underwent surgery, among which 750 patients had lobectomy, 116 patients had wedge resection and 36 patients had segmentectomy. The most prevalent histological type was adenocarcinoma which was confirmed in 830 cases (87.7%). And adenocarcinoma was invasive in 699 patients (84.2%) with acinar predominant tumor [369 cases (52.8%)] was the most common subtype. Other subtype of invasive adenocarcinoma included lepidic predominant tumors in 163 cases (23.3%), solid predominant tumors in 67 cases (9.6%), papillary predominant tumors in 62 cases (8.9%), mucinous adenocarcinoma in 24 cases (3.4%) and micropapillary predominant tumors in 14 cases (2.0%). The other 54 cases (6.5%) had no definite histological subtypes of adenocarcinoma. There are also 194 cases (21.5%) of visceral pleura invasion and 50 cases (5.5%) of lymphovascular invasion. 838 patients underwent lymph node dissection or sampling, among which 129 patients (15.4%) had lymph node metastasis. N1 and N2 lymph node involvement occurred in 54 patients (6.4%) and 75 patients (8.9%) respectively. Pathological stage I, II, III and IV tumors were found in 692 (80.0%), 52 (6.0%), 73 (8.4%), and 48 (5.5%) patients respectively. In addition, EGFR gene mutation was found in 59.3% patients (324 of 546).
Clinicopathologic characteristics between cavitary lung cancer and noncavitary lung cancer
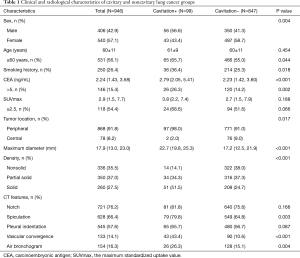
The clinical and radiological characteristics were listed in Table 1. Comparing with noncavitary lung cancer, cavitary lung cancer occurred more frequently in elderly (P=0.044), male (P=0.004), who had a history of smoking (P=0.018) and higher CEA level (P<0.001). In addition, cavitary lung cancer were significantly associated with the presence of several radiological features, including peripheral nodules (P=0.017), maximum tumor diameter (P<0.001), solid nodules (P<0.001), spiculation (P=0.003), vascular convergence (P<0.001) and air bronchogram (P=0.004). On CT images, separations within cavities (44 cases, 44.4%) and blood vessels passing through the cavities (20 cases, 20.2%) was found in cavitary lung cancer.
Full table
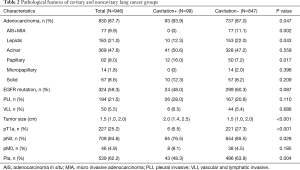
Histologic findings reveals that there are larger tumor size (P<0.001), advanced T stage (P<0.001), more lymph node metastasis (P=0.028), advanced pathological staging (P=0.004), more adenocarcinoma (P=0.047) and more papillary predominant tumors (P<0.001) in patients with cavitary lung cancer. While, noncavitary lung cancer occurred more frequently in AIS/MIA (P=0.002) and lepidic predominant tumors (P<0.001). In addition, there were no significant differences in visceral pleura invasion, LVI and EGFR gene mutation status between the two groups (Table 2).
Full table
Multivariable analysis for the correlation between clinicopathological characteristics and cavitation
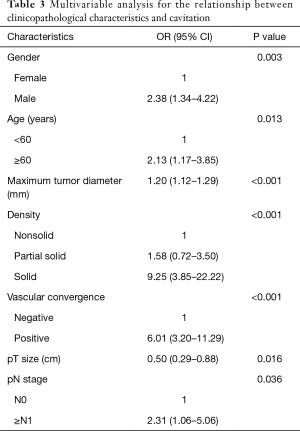
Multivariable analysis showed that cavitation was significantly associated with elderly (OR =2.13, 95% CI: 1.17–3.85, P=0.013), male (OR =2.38, 95% CI: 1.34–4.22, P=0.003), larger maximum tumor diameter (OR =1.20, 95% CI: 1.12–1.29, P<0.001), solid nodules (OR =9.25, 95% CI: 3.85–22.22, P<0.001), larger pT size (OR =0.50, 95% CI: 0.29–0.88, P=0.016) and advanced pN stage (OR =2.31, 95% CI: 1.06–5.06, P=0.036) (Table 3).
Full table
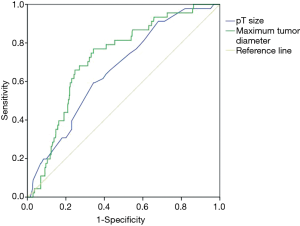
ROC curve was used to determine optimal cut-off values for continuous variables (Figure 2). The AUC of maximum tumor diameter was 0.71 (95% CI: 0.66–0.75, P<0.001), a cut off value of 20.9 mm showed a discriminatory power of cavitation with a sensitivity of 68.7% and a specificity of 71.2%. The AUC of pT size was 0.66 (95% CI: 0.61–0.71, P<0.001), a cut off value of 2.0 cm showed a discriminatory power of cavitation with a sensitivity of 59.3% and a specificity of 65.6%.
Discussion
In the current study in which 99 patients with cavitary lung cancer were enrolled, incidence of cavitary lung cancer was 10.5%, which was similar to previous reports (3). Recently, it was reported that the incidence of cavitary lung adenocarcinoma was about 6.2% (11), which was consistent with the results of our study (9.8%). It has been reported that patients with cavitary lung cancer presented more in male patients and patients with smoking history than patients with noncavitary lung cancer (11). In our study, cavitary lung cancer occurred more frequently in elderly (P=0.013) and male (P=0.003), but not in patients who have a history of smoking. The proportion of smoking history in cavitary lung cancer patients is relatively low, only 36.4%, while it has been reported that just the proportion of smoking history in cavitary lung adenocarcinoma was as high as 66.4% (11). We suggest that it was associated with the inclusion of more female patients (57.1%), as Asian women smoke less but are more susceptible to the effects of passive smoking.
In previous studies, both cavitary squamous cell carcinoma and cavitary adenocarcinoma had larger tumor size and advanced T staging (11-13). Similarly, we found that cavitary lung cancer had larger maximum tumor diameter (P<0.001) and larger pT size (P=0.016) in our cohort. ROC curve showed that maximum tumor diameter had stronger discriminatory power than pT size in predicting cavitation. A cut off value of 20.9 mm showed a discriminatory power of cavitation with a sensitivity of 68.7% and a specificity of 71.2%. We suggest that it was related to the pathogenesis of cavitary lung cancer. Zhang et al. (14) used digital scanner to detect the number and area of blood vessels in tumor tissues of 105 patients with non-small cell lung cancer, and found that the number of blood vessels decreased with the increase of tumor size, but the area of blood vessels within the tumor remained stable. Therefore, as the tumor tissue grows, the irregular distribution of blood flow in the tumor will lead to ischemia and hypoxia of the partial tissue. After the necrosis of the ischemic tissue, it will be discharged through the drainage bronchus, forming a cavitation.
The findings of CT images revealed that there were more vascular convergences (P<0.001) in cavitary lung cancer patients. In addition, it also had some characteristic performance, including the blood vessels passing through the cavities and the separations within cavities. These signs were mostly seen in thin-walled cavities, and closely related to its formation mechanism. With the improvement of people’s understanding of cavitary lung cancer, the reports of thin-walled cavitation lung cancer gradually increased (15-17). However, its exact mechanism remains unclear, and currently, most scholars are in favor of the check-valve mechanism. Tumor cells grow along the alveolar walls or shed and block the bronchioles. Due to the lack of cartilage in the bronchioles, tumor cells play an indirect role as a valve. With the destruction of the alveolar wall by tumor cells and the gradual increase of air in the alveolar cavity, the alveolar wall ruptures and fuses with each other to form the thin-walled cavity (18). The separations within the cavitation are the alveolar septal tissues that have not yet been completely destroyed, which had been confirmed in pathology (19). There are relatively few reports on the sign of blood vessels passing through cavitation. One study speculated that the possible cause of formation is the contraction of elastic fibers in tumor tissues, which leads to the formation of cavitation wall cracks and allows small blood vessels to enter the cavitation through local defects (20). However, there is currently no comparison between radiology and pathology.
With regard to tumor density on CT, it is—to our knowledge—the first report showing that cavitary lung cancer present as more solid nodules on CT, while noncavitary lung cancer had more nonsolid components (P<0.001). This characteristic of CT image of cavitary lung cancer was consistent with the pathological subtype of cavitary lung adenocarcinoma. When ground glass opacity (GGO) was shown on CT, the pathological subtypes of adenocarcinoma are mostly AIS, MIA and lepidic predominant invasive adenocarcinoma (21). This was also confirmed in the present study, cavitary lung cancer was more common in invasive adenocarcinoma, especially the papillary type (P=0.017), while noncavitary lung cancer occurred more frequently in AIS/MIA (P=0.002) and lepidic predominant (P=0.043), which was basically consistent with the report of Watanabe et al. (11). They also found that there were more solid predominant cases in cavitary lung cancer, but there was no significant difference in AIS and MIA between cavitary and noncavitary lung cancer. In addition, the most common histological subtype of cavitary lung invasive adenocarcinoma was papillary type (43.2%), while in our study it was acinar type (51.2%), which was similar to the study of Tomizawa et al. (22). We suggest that it could be due to the difference inclusion criteria among studies, our study and Tomizawa’s study included more early patients, so the relationship between cavitary lung adenocarcinoma and pathological subtypes needs to be confirmed by further studies. The consistency of CT manifestations and pathological subtypes of cavitary lung adenocarcinoma indicates that cavitary lung adenocarcinoma tends to have a poor prognosis, which has been confirmed in recent studies. The study of Watanabe et al. showed that cavitation was an independent prognostic risk factor for adenocarcinoma (11). However, due to the lack of patient survival data, the clinicopathological features of cavitary lung cancer cannot be verified.
More EGFR mutations have been reported in noncavitary lung adenocarcinoma, with a mutation rate of 47.3% and 33.6% respectively (11). In the present study, no significant difference was found between lung adenocarcinoma with cavitation and without cavitation in EGFR mutations status, but the mutation rate of EGFR gene was significantly higher than that of previous study (23). It may be related to the fact that our study was a retrospective study and EGFR gene tests were not conducted in the entire cohort, with only 57.5% of patients were tested for EGFR gene mutations. Furthermore, one research has shown that cavitary lung adenocarcinoma was common in pleural invasion, vascular and lymphatic invasion and lymph node metastasis (11). However, the findings of our study provide that there are more lymph node metastasis (P=0.036) in cavitary lung cancer, but there are no significant difference in VPI and lymphovascular invasion. Considering that the samples included in this study are mainly early-stage tumors with relatively few occurrences, it is necessary to confirm whether there are more pleural invasion and vascular infiltration of cavitary lung cancer in patients with early-stage lung cancer by large sample studies.
In our study, 217 patients underwent PET-CT examination, and the results showed that no significant difference in SUVmax between cavitary lung cancer and noncavitary lung cancer (P=0.188). It may be related to the relatively small sample in the study (22.9%) or the existence of cavitation. A partial volume effect between the cavity and the tumor tissue can lead to a relative decrease in lesion uptake. In addition, the presence of GGO components also has a certain impact on the measurement of uptake values. Most of them are pathological components with low invasive degrees, such as AIS, MIA and lepidic predominant invasive adenocarcinoma. These tumor components grow slowly and have low metabolic activity, leading to relatively low uptake in PET-CT (21,24). Therefore, PET-CT is not a sensitive detection method for ≤3 cm cavitary lung cancer, especially the lung cancer with large cavity and GGO components. However, its advantages in assessing systemic metastasis are of great significance in determining cavitary lung cancer, especially thin-walled cavitary lung cancer which is difficult to distinguish with other benign pulmonary cavitary diseases on CT.
This study has several limitations. As a monocenter retrospective study, selection bias was unavoidable and the incidence of cavitary lung cancer was not assessed adequately. Because of its retrospective design, some data were also missing, especially data regarding the SUVmax and EGFR gene mutation status. In addition, this study lacks the correlation analysis of patient survival and recurrence, cannot verify the worse prognostic clinicopathological features.
Conclusions
The present study confirms that cavitary lung cancer has some worse prognostic clinical, radiological and pathological characteristics, comparing with noncavitary lung cancer. Especially, cavitary lung cancer present as more solid nodules on CT images and present with more invasive on pathological findings.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-426
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-426). DL serves as an unpaid editorial board member of Journal of Thoracic Disease from Nov 2016 to Oct 2020. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by our institutional ethics board of China-Japan Friendship Hospital (No. 2018-13-K08).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Chaudhuri MR. Primary pulmonary cavitating carcinomas. Thorax. 1973;28:354-66. [Crossref] [PubMed]
- Koenigkam Santos M, Muley T, Warth A, et al. Morphological computed tomography features of surgically resectable pulmonary squamous cellcarcinomas: impact on prognosis and comparison with adenocarcinomas. Eur J Radiol 2014;83:1275-81. [Crossref] [PubMed]
- Onn A, Choe DH, Herbst RS, et al. Tumor Cavitation in Stage I Non-Small Cell Lung Cancer: Epidermal Growth Factor Receptor Expression and Prediction of Poor Outcome. Radiology 2005;237:342-7. [Crossref] [PubMed]
- Gasinska A, Kolodziejski L, Niemiec J, et al. Clinical significance of biological differences between cavitated and solid form of squamous cell lung cancer. Lung Cancer 2005;49:171-9. [Crossref] [PubMed]
- Shimosato Y, Suzuki A, Hashimoto T, et al. Prognostic implications of fibrotic focus (scar) in small peripheral lung cancers. Am J Surg Pathol. 1980;4:365-73. [Crossref] [PubMed]
- Collins J, Stern EJ. Ground-glass opacity at CT: the ABCs. AJR Am J Roentgenol 1997;169:355-67. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Watanabe Y, Kusumoto M, Yoshida A, et al. Surgically Resected Solitary Cavitary Lung Adenocarcinoma: Association Between Clinical, Pathologic, and Radiologic Findings and Prognosis. Ann Thorac Surg 2015;99:968-74. [Crossref] [PubMed]
- Mouroux J, Padovani B, Elkaim D, et al. Should cavitated bronchopulmonary cancers be considered a separate entity? Ann Thorac Surg 1996;61:530-2. [Crossref] [PubMed]
- Coffey JP, Hill JC. 18F-Fluoro-2-deoxy-D-glucose standardized uptake value in cavitating non-small-cell lung carcinoma. Nucl Med Commun 2008;29:1040-5. [Crossref] [PubMed]
- Zhang L, Yankelevitz DF, Henschke CI, et al. Variation in vascular distribution in small lung cancers. Lung Cancer 2010;68:389-93. [Crossref] [PubMed]
- Fintelmann FJ, Brinkmann JK, Jeck WR, et al. Lung Cancers Associated With Cystic Airspaces: Natural History, Pathologic Correlation, and Mutational Analysis. J Thorac Imaging 2017;32:176-88. [Crossref] [PubMed]
- Mascalchi M, Attinà D, Bertelli E, et al. Lung cancer associated with cystic airspaces. J Comput Assist Tomogr 2015;39:102-8. [Crossref] [PubMed]
- Xue XY, Liu YX, Wang KF, et al. Computed tomography for the diagnosis of solitary thin-walled cavity lung cancer. Clin Respir J 2015;9:392-8. [Crossref] [PubMed]
- Aronberg DJ, Sagel SS, Lefrak S, et al. Lung carcinoma associated with bullous lung disease in young men. AJR Am J Roentgenol 1980;134:249-52. [Crossref] [PubMed]
- Farooqi AO, Cham M, Zhang L, et al. Lung Cancer Associated With Cystic Airspaces. AJR Am J Roentgenol 2012;199:781-6. [Crossref] [PubMed]
- Goto T, Maeshima A, Oyamada Y, et al. Cavitary Lung Cancer Lined with Normal Bronchial Epithelium and Cancer Cells. J Cancer 2011;2:503-6. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Tomizawa K, Shimizu S, Ohara SC, et al. Clinical significance of tumor cavitation in surgically resected early-stage primary lung cancer. Lung Cancer 2017;112:57-61. [Crossref] [PubMed]
- Watanabe Y, Kusumoto M, Yoshida A, et al. Cavity Wall Thickness in Solitary Cavitary Lung Adenocarcinomas Is a Prognostic Indicator. Ann Thorac Surg 2016;102:1863-71. [Crossref] [PubMed]
- Ambrosini V, Nicolini S, Caroli P, et al. PET/CT imaging in different types of lung cancer: an overview. Eur J Radiol 2012;81:988-1001. [Crossref] [PubMed]