This article has an erratum available at: http://dx.doi.org/10.21037/jtd-2021-04 and the article has been update on 2021-01-14 at here.
The role of postoperative radiotherapy for thymomas: a multicentric retrospective evaluation from three Italian centers and review of the literature
Introduction
Thymic epithelial tumors represent a heterogeneous group of rare thoracic cancers, with an annual incidence ranging from 1.3 to 3.2 per million (1). Thymoma is the most common primary neoplasm of the anterior mediastinum. The etiology of thymomas is unknown. Mean age at diagnosis is 40–70 years (2). According to the World Health Organization (WHO) histopathological classification, thymomas are distinguished in different types: A, AB, B1, B2, B3 (3,4).
Thymoma is characterized by an indolent growth pattern. Nevertheless, local invasion, pleural dissemination, and metastases can occur (5). The most important determinants of long-term survival in thymoma are the Masaoka stage, completeness of resection, and histologic classification (6-9).
Most of the patients are asymptomatic, but symptoms could be chest pain, cough, or dyspnea. Autoimmune disorders like myasthenia gravis affect about one-third of patients with thymoma (10). Other syndromes more often associated with thymoma are pure red cell aplasia (5%) and Good’s syndrome (5%) (11,12).
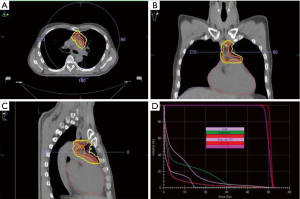
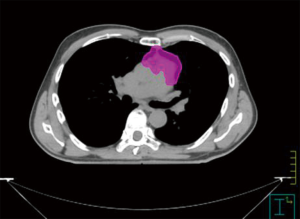
Complete surgical resection is the backbone in the treatment of early stage and locally advanced thymoma, and the extent of resection is an independent prognostic factor of improved survival (13,14). Recurrence rates after surgery are 10%, 30% and 60% in Masaoka-Koga I/II, stage III and stage IV thymoma, respectively (15). Stage, completeness of resection [microscopic negative margin (R0), microscopic positive margin (R1), macroscopic positive margin (R2)] and histology are key prognostic criteria, even if the histologic subtype is less important than stage and the extent of resection (16-19). These factors guide the decision whether to perform postoperative radiotherapy (PORT) or not. Radiotherapy after surgery should be performed within at least 3 months from surgery. Generally, PORT is strongly recommended for incompletely resected thymomas (20,21). Radiotherapy must be delivered based on a 3D conformal technique or intensity-modulated radiotherapy (IMRT) directed to the tumor bed (Figure 1). The clinical target volume (CTV) should include the whole thymic space, the tumor and its extensions in the anterior, superior and middle mediastinum. Involved nodes and the resected pleural implant can be included in the treated volumes (Figure 2) (22). Extensive elective nodal irradiation is no longer recommended, because metastasis to regional lymph nodes are uncommon (23,24). A recent retrospective evaluation avoids this recommendation (25). The fractionated total dose after a R0 resection should be of 45–50 Gy, with a daily dose of 1.8–2 Gy over a 4- to 6-week period. Doses of 50–54 Gy can be applied after a R1 resection, with a boost dose to areas of likely residual disease. The unresectable disease requires a total dose of 60 to 70 Gy.
With the present study, we aimed to assess the efficacy and safety of PORT. Moreover, we want to identify the predictive factors on survival after PORT, in order to distinguish patients who may benefit more from adjuvant radiation treatment. Finally, we reviewed the recent literature on the role of PORT in resected thymoma. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-2019-thym-09).
Methods
We retrospectively analyzed a series by three different Institutions, University Hospital “Policlinico” of Modena (Modena), University and “Spedali Civili” of Brescia (Brescia), University Hospital “Careggi” (Florence). The study protocol was reviewed and approved by the Ethics Committee of the Coordinator Center in Modena.
All thymomas were classified according to both the WHO histologic classification (26) and the Masaoka clinical staging system (23). Acute and late adverse events (AEs) were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
We analyzed selected variables of patients characteristics: age, sex, symptoms, ECOG performance status (PS) and description of paraneoplastic syndrome (if present). Kind of procedure, execution of lymphadenectomy and resected radical margins (R0, R1 or R2) were the variables of interest for surgery. We also collected data on technique, dose and fractionation of radiation therapy. Data on local or distant relapse were evaluated, as well as those collected from the last follow up.
All patients underwent surgery with a curative intent and radical resection was the aim of surgery. Most operations were conducted through a median sternotomy. Thymectomy, instead of simple thymomectomy or tumor enucleation, was the standard procedure. In patients with myasthenia gravis, an extended thymectomy was generally performed. Lateral or anterolateral thoracotomy was chosen, according to the surgeon’s preference, in case of small lateralized tumors in non-myasthenic patients. Video-assisted thoracoscopic approach was rarely performed, according to the surgeon’s preferences and experience, in cases of early stage/capsulated thymomas. Mediastinal lymph node dissection was not routinely performed. Myasthenic patients were routinely admitted to the intensive care unit for at least 24 hours, for postoperative care. An R0 resection was defined by the absence of tumor on the margins of resection, or when lesion microscopically involved an area where no further tissue could be resected; R1 is defined by resection the permanence of microscopic residual tumor cells, while a R2 removal described when macroscopic residual tumor occurred after surgery.
Indication to PORT included close or positive margins or incompletely resected invasive thymoma. Moreover, patients with unfavorable features (advanced Masaoka stage at diagnosis, worse WHO cells classification) were generally addressed to radiation therapy after surgery.
The follow up schedules were similar in all the three institutions. After primary treatment, follow-up procedures included CT scan of the thorax and upper abdomen every 3–6 months for the first 2 years, then once a year for a maximum of 10 years. After the end of the follow-up schedule, follow-up continued by telephone calls for a clinical update. Patients who interrupted follow-up and did not answer the phone calls were considered lost to follow-up.
Statistical analysis
Descriptive data were expressed in terms of frequency, mean and standard deviation. Frequencies were compared using the χ2-test or Fischer’s exact test for categorical variables. Continuous variables were compared using the t-test. All the variables were compared to each other in a univariate analysis to find associations. Multivariate analysis of survival was performed using Cox regression model and hazard ratios (HR) with 95% confidence interval (CI) were calculated. Survival curves were calculated according to the Kaplan-Meier method and comparisons were performed using the log-rank test. OS, DSS and DFS were investigated. Continuous variables were dichotomized to estimate odds ratios (OR) when needed and to include them in the multivariate analysis; the median value of the whole population was used as the cut-off value. Variables with more than two categories were converted in binomial variables, in order to achieve more reliable results in the univariate analysis and to perform the multivariate analysis. ECOG performance status 1 and 2 were grouped. Regarding WHO cell type, two different binomial variables were created for the analysis: A + AB + B1 versus B2 + B3 + C and A + AB versus B1 + B2 + B3 + C. Also regarding Masaoka staging, two different binomial variables were created: stage I + II versus stage III + IV and stage I versus stage II + III + IV.
A value of P<0.05 was considered significant.
All analyses were performed using SPSS 25.0 statistical software (SPSS Inc., Chicago, IL, USA).
Results
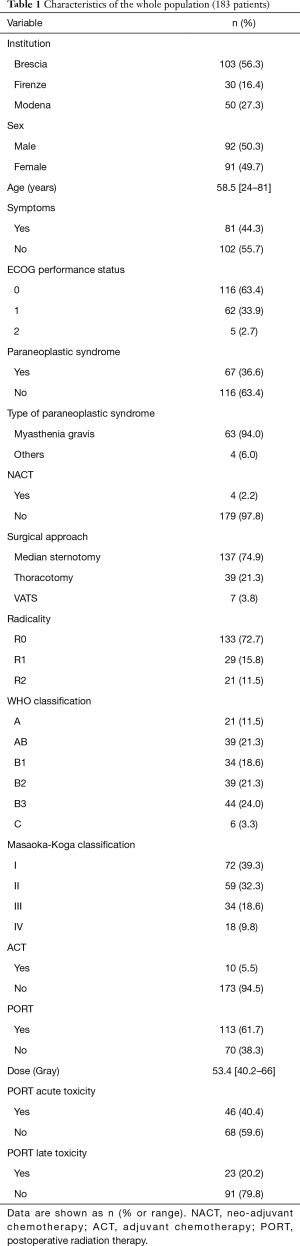
From 1981 to 2015, 183 consecutive patients underwent surgery for diagnosis of thymoma. Table 1 shows the clinical and pathological characteristics of the whole population. A very small number of patients underwent induction or adjuvant chemotherapy, therefore these variables were not included in the statistical analysis.
Full table
Patients
Median age was 58.5 (range, 24–81) years. Paraneoplastic syndrome was present in 67 patients (59.2%), 63 of whom had myasthenia gravis. According to the Masaoka-Koga staging system, 72 patients were (39.3%) in stage I, 59 patients (32,5%) in stage II, 34 patients in stage III patients (18,5%) and 18 in stage IV (9.7%). At the univariate analysis, patients with advanced Masaoka stage presented poorer PS (P=0.021, OR 2.12, 95% CI: 1.11–4.09). Patients suffering from paraneoplastic syndrome had poorer PS (P<0.001, OR 3.04, 95% CI: 1.62–5.72), had more aggressive WHO type tumors (P<0.001, OR 3.84, 95% CI: 1.82–8.09) and more advanced Masaoka stage tumors (P=0.015, OR 2.20, 95% CI: 1.15–4.19). Symptomatic patients presented a poorer PS compared to asymptomatic patients (P<0.001, OR 6.35, 95% CI: 3.25–12.35). There was a strong correlation between WHO cell type and Masaoka staging: tumors presenting with aggressive WHO cell type (B2 + B3 + C) were found to have a more advanced stage (II–IV) (P<0.001, OR 8.82, 95% CI: 3.95–19.70). Twelve patients with type A tumor (57%) were in stage I, while only 2 (9.5%) were in stage IV.
Surgery
Median sternotomy was the most common surgical procedure (n=137, 74,9%). Surgery resulted in complete resection in 133 patients (72.7%), whereas microscopic persistence of disease (R1) was present in 29 patients (15.8%) and a macroscopic persistence in 21 patients (11.5%). The overall univariate analysis showed the following significant associations among variables. Compared with younger patients, older ones were diagnosed with poorer PS (mean age 56.4 versus 62.0 years for PS 0 and 1+2 patients, respectively; P=0.008), and more frequently underwent thoracotomy (mean age 62.5 versus 57.2 for patients who underwent thoracotomy and sternotomy, respectively; P=0.013). Moreover, older patients had less aggressive WHO type tumors (mean age 62.4 versus 54.4 for WHO A + AB + B1 and B2 + B3 + C respectively, P<0.001). Patients suffering from paraneoplastic syndrome more frequently underwent median sternotomy (P=0.012, OR 2.64, 95% CI: 1.21–5.75). Incomplete resection occurred more often in patients with advanced Masaoka stage compared to patients with early stage tumors (P<0.001, OR 4.87, 95% CI: 2.12–11.15). No relationship was found between WHO cell type and completeness of resection.
PORT
PORT was delivered in 114 patients (62.3%), while the remaining 69 subjects underwent surgery alone. Among the 114 patients undergoing radiation therapy, 25% (n=28) were in stage I, 36% (n=41) in stage II, 26% (n=29) in stage III, and 13% (n=15) in stage IV. Complete resection was obtained in 68 patients, while the remaining 45 had positive margins.
WHO classification for these patients was A, AB, B1, B2, B3, C in 6, 20, 21, 27, 34 and 6 patients, respectively. Radiotherapy was delivered with a total dose ranging between 40 and 66 Gy using conventional fractionation (1.8–2 Gy, daily), based on the state of surgical margins. The PTV encompassed the mediastinal surgical bed with the inclusion of all surgical clips and a boost dose was delivered on the macroscopic disease, if present. Radiation therapy was administered with 2D technique in 35 patients, while 66 patients were treated with 3D-CRT. The remaining 12 patients were treated with VMAT (n=10) and IMRT (n=2). At the univariate analysis, patients with incomplete resection more frequently underwent PORT (P<0.001, OR 10.99, 95% CI: 3.74–32.26) and were treated with a higher dose of radiation (mean dose 55.7 versus 51.9 respectively, P<0.001) compared to patients who obtained a complete resection. PORT was more frequently performed in patients with aggressive WHO cell type tumors (P<0.001, OR 3.28, 95% CI: 1.72–6.25) and with advanced Masaoka stage (P<0.001, OR 4.84, 95% CI: 2.54–9.23). Finally, patients who had suffered from acute PORT toxicity had a higher risk to develop late toxicity than patients who did not (P=0.025, OR 2.86, 95% CI: 1.11–7.35).
Survival
Mean follow-up was 130 months (standard deviation 82, range 3–417 months). At the end of follow-up, 98 (53.6%) patients were alive without evidence of disease, 7 (3.8%) were alive with recurrence, 21 (11.5%) were dead with recurrence and 57 (31.1%) were dead for causes other than the tumor. The first site of recurrence was loco-regional in 10 patients (36%), distant in 14 patients (50%) and both loco-regional and distant in 4 patients (14%). OS of the whole population at 3, 5 and 10 years from surgery was 95.6%, 90.2% and 69.7% respectively; median OS time was 199.9 months. Three-, 5- and 10-year DSS was 97.8%, 92.3% and 89.8% respectively. DFS at 3, 5 and 10 years from surgery was 93.3%, 88.3% and 82.8% respectively (Figures 3-5).
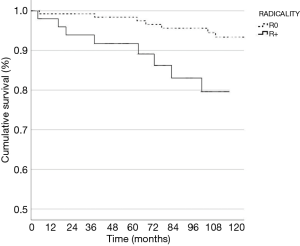
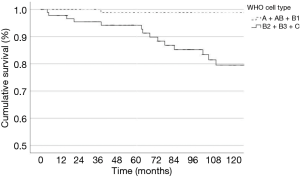
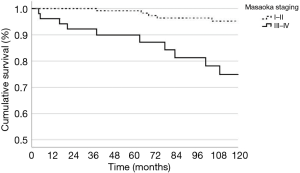
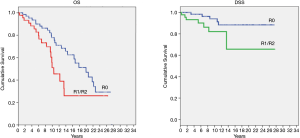
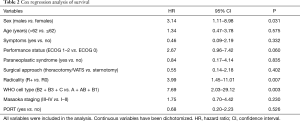
When treated with PORT, a trend towards a better OS was evident for patients in Masaoka stage I–II versus patients in stage III–IV (P=0.09). Complete resection resulted in a better OS upon positive margins (R1 and R2) when PORT was delivered (P=0.05). However, after radiation therapy, patients with completely resected disease had a better DSS compared to R1/R2 (P=0.04) (Figure 6). We found a significant difference on DSS for gender, with an advantage for female patients undergoing PORT (P=0.03). In terms of DSS no differences for PS (P=0.70), WHO histology (P=0.19), paraneoplastic syndrome at diagnosis (P=0.23) and surgical procedure (P=0.53) were found. Table 2 reported Cox regression analysis of survival.
Full table
In terms of clinical outcomes, univariate analysis showed that complete resection, A + AB + B1 cell type and stage I–II were significant predictor factors of better DSS, as well as DFS. The rates of death for progression of disease was 0% for A cell type, 2.5% for AB cell type (2.5%) and 83% for C cell type tumor. Female patients had significantly better tumor-specific survival than males: 98.9% and 95.3% versus 93.0% and 84.1% at 5 and 10 years from surgery, respectively (P=0.024). Of note, PORT did not affect survival and PORT toxicity did not have a negative impact on survival.
Multivariate analysis of survival showed that gender, total resection and WHO cell type remained independent prognostic factors, while Masaoka staging did not.
Toxicity
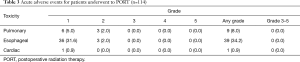
Among 70 patients undergoing surgery alone, only 1 had an acute AE (grade 1 cardiac toxicity). Patients treated with PORT had a higher level of acute AEs: 45 (39.5%) experienced any grade toxicity from treatment, but none of these was graded ≥3.
Acute toxicities for patients treated with PORT were reported in Table 3.
Full table
Discussion
The role of PORT in patients with thymoma after complete resection remains controversial and the approach is different depending on the Masaoka-Koga stage of disease. Due to the rarity of the disease, there is a lack of randomized and prospective trials that can lead to level I evidence indications; consequently, the optimal role of PORT remains based mainly on retrospective studies. Besides, the results of some studies on thymoma are inconclusive due to the heterogeneity of cohorts analyzed.
In patients with completely resected stage I tumors, PORT has not been recommended because of the low incidence of local recurrence rates following complete resection (27-31). In early stage thymoma, PORT is only suggested in high-risk situations, such as an incomplete resection. Liu et al. conducted a retrospective analysis of 1,500 patients with stage I to III thymic tumors included in the Chinese Alliance for Research in Thymoma (ChART) database and found that an incompletely resected thymoma or thymic carcinoma could have a survival benefit from PORT, while a R0 resected did not (32). The optimal management of patients presenting with stage II tumors remains controversial (20). Several studies supported the use of PORT when macroscopic capsular invasion (stage IIB) is reported, but not in stage IIA (29,33). Patients in stage III–IV with complete resection have higher risks of relapse. A potential OS benefit in receiving PORT versus surgery alone in stage III–IV disease—but not in stage II disease—was evident in the meta-analysis of Lim et al. The results of this meta-analysis showed that PORT had no effect on survival in patients with Masaoka stage I–II disease (HR 1.45, 95% CI: 0.83–2.55, P=0.2), but a survival benefit was observed in stage III–IV (HR 0.63, 95% CI: 0.40–0.99, P=0.04) with even better results if older trials were excluded from the analysis (HR 0.54, 95% CI: 0.33–0.88, P=0.01) (7). Another meta-analysis analyzing 14 studies conducted from 1996 to 2014 found an OS benefit of PORT in completely resected stage II and III thymoma (HR 0.57 and 0.73, respectively), but no advantages were seen in DSS and DFS (34). To emphasize the lack of clarity in this setting, a third meta-analysis conducted on retrospective trials from 1984 to 2014 found no benefit on recurrent outcomes in either the stage II or the stage III R0 patients (35) (Table 4).

Full table
Other studies were published after these meta-analyses. One propensity score-matched analyses conducted recently reported a survival advantage for PORT in stage IIB thymoma (HR =0.61), stage III (HR =0.69), and positive margins (HR =0.53), but not for stage I to IIA (33). The same benefit was observed in a retrospective ITMIG evaluation of completely resected stage III thymoma, in which 5- and 10-year OS rates were 95% and 86%, respectively, compared to 90% and 79% for patients receiving surgery alone (P=0.002) (36). Liao et al. collected data on completely resected stage III thymoma, showing a benefit in the 5- and 8-year OS, with rates of 95.6% and 93.9% with PORT; the same endpoints for surgery alone were 84.0% and 67.2%, respectively (P=0.004) (37). Interestingly, one recent retrospective analysis on the Surveillance, Epidemiology, and End Results (SEER) database evaluated 2,234 patients from 1988 to 2013 confirmed the OS and DSS benefit of PORT with stage III/IV disease, but adjuvant radiotherapy decreased the DSS for those with stage I/IIA thymoma. The improvement in OS and DSS in patients treated with PORT when a capsular infiltration occurs was found in other retrospective experiences (38-40). The most recent retrospective studies on PORT are reported in Table 5.

Full table
Some importance in choosing PORT for patients is also given to the clinical characteristics. We observed a survival advantage in histologic WHO subtype A, AB and B1, and this finding is in line with the results of two National Cancer Data Base (NCDB) studies and with a retrospective evaluation (33,37,41). Another study suggests that subtype B1, B2, B3 benefit more from PORT (36). Also completeness of resection (33,42) is associated with a greater OS, and this corroborates our findings (33,40,42). Some evaluations reported that a higher Masaoka-Koga stage is detrimental for survival (29,33,36), but our results deviate from this. We observed that age at diagnosis is not a prognostic factor, while other studies report that older age is associated with a poor outcome (29,33,36,42). Multivariate analysis showed that PORT did not impact survival, and this result was confirmed by Weksler et al. (39). Interestingly, one series showed a survival improvement in diagnosis made in more recent years, likely due to advances in surgical and radiation techniques and improvements in patient selection (29).
We did not find a higher rate of both grade ≥3 acute and late AEs in patients treated with PORT, and the absence of an increased rate in cardiac mortality with PORT was also evident in literature (29). Our result should be correlated with the findings of Liao et al., that observed that higher heart dose was related to increased risk of cardiovascular disease in long- term survivors (37). The rate of low grade acute AEs were higher with PORT compared to surgery alone, even if all the patients recovered completely from toxicities and showed no late consequences from these events.
This study had several limitations. First of all, this is a retrospective analysis and carries with it all the limitations of a retrospective evaluation. Secondly, follow-up schedules were not standardized and this can lead to bias toward null hypotheses. In addition, we could not evaluate the influence of postoperative chemotherapy because there were only a few patients who underwent it. Another limitation is the number of 2-D treatments: up to date this technique is obsolete for PORT in thymoma. Finally, our retrospective analysis collected data from a large number of patients in stage I–II (n=69) compared to patients in stage III–IV (n=44); this difference may have altered the results of effectiveness in more advanced stages of thymoma.
In conclusion, the review of the literature showed that PORT provides a significant benefit in selected patients affected by thymoma in terms of OS, DSS and DFS. This advantage was evident in stage IIb–IV thymoma, whilst patients in early stage disease didn’t have the same benefit. Surgery alone was confirmed as the standard of care in stage I–IIa thymoma. Our results demonstrated that PORT is highly recommended in patients with incomplete resection (R1 and R2 margins). From our analysis, an important finding is that PORT didn’t increase high grade acute toxicity compared to surgery alone, and this result is evident both in our evaluation and in the other retrospective experiences. Despite the difficulty to conduct prospective randomized trials due to the low incidence of disease, controlled studies are necessary to confirm the role of PORT for stage I–IV thymoma.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Dragana Jovanovic and Semra Bilaceroglu) for the series “Thymoma” published in Journal of Thoracic Disease. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-2019-thym-09
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-2019-thym-09
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-2019-thym-09). The series “Thymoma” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was reviewed and approved by the Ethics Committee of the Coordinator Center in Modena.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- de Jong WK, Blaauwgeers JLG, Schaapveld M, et al. Thymic epithelial tumors: A population-based study of the incidence, diagnostic procedures and therapy. Eur J Cancer 2008;44:123-30. [Crossref] [PubMed]
- Detterbeck FC, Zeeshan A. Thymoma: Current diagnosis and treatment. Chin Med J (Engl) 2013;126:2186-91. [PubMed]
- Travis WD, Müller-Hermelink HK BE. Pathology and Genetics: Tumours of the Lung, Pleura, Thymus and Heart. Int Agency Res Cancer 2004.
- Marx A, Ströbel P, Badve SS, et al. ITMIG consensus statement on the use of the WHO Histological classification of thymoma and thymic carcinoma: Refined definitions, histological criteria, and Reporting. J Thorac Oncol 2014;9:596-611. [Crossref] [PubMed]
- Girard N, Mornex F, Van Houtte P, et al. Thymoma: A focus on current therapeutic management. J Thorac Oncol 2009;4:119-26. [Crossref] [PubMed]
- Safieddine N, Liu G, Cuningham K, et al. Prognostic factors for cure, recurrence and long-term survival after surgical resection of thymoma. J Thorac Oncol 2014;9:1018-22. [Crossref] [PubMed]
- Lim YJ, Kim E, Kim HJ, et al. Survival Impact of Adjuvant Radiation Therapy in Masaoka Stage II to IV Thymomas: A Systematic Review and Meta-analysis. Int J Radiat Oncol Biol Phys 2016;94:1129-36. [Crossref] [PubMed]
- Singhal S, Shrager JB, Rosenthal DI, et al. Comparison of Stages I-II Thymoma Treated by Complete Resection with or Without Adjuvant Radiation. Ann Thorac Surg 2003;76:1635-41. [Crossref] [PubMed]
- Lee GD, Kim HR, Choi SH, et al. Prognostic stratification of thymic epithelial tumors based on both Masaoka-Koga stage and WHO classification systems. J Thorac Dis 2016;8:901-10. [Crossref] [PubMed]
- Evoli A, Lancaster E. Paraneoplastic disorders in thymoma patients. J Thorac Oncol 2014;9:S143-7. [Crossref] [PubMed]
- Means RT Jr. Pure red cell aplasia. Hematology Am Soc Hematol Educ Program 2016;2016:51-6. [Crossref] [PubMed]
- Jansen A, van Deuren M, Miller J, et al. Prognosis of Good syndrome: mortality and morbidity of thymoma associated immunodeficiency in perspective. Clin Immunol 2016;171:12-7. [Crossref] [PubMed]
- Ströbel P, Bauer A, Puppe B, et al. Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: A retrospective analysis. J Clin Oncol 2004;22:1501-9. [Crossref] [PubMed]
- Ried M, Marx A, Götz A, et al. State of the art: Diagnostic tools and innovative therapies for treatment of advanced thymoma and thymic carcinoma. Eur J Cardiothorac Surg 2016;49:1545-52. [Crossref] [PubMed]
- Detterbeck FC. Towards a TNM based prognostic classification for thymic tumours. J Thorac Oncol 2014;9:S65-72. [Crossref] [PubMed]
- Ogawa K, Toita T, Uno T, et al. Treatment and prognosis of thymic carcinoma a retrospective: Analysis of 40 cases. Cancer 2002;94:3115-9. [Crossref] [PubMed]
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: A clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84. [Crossref] [PubMed]
- Ruffini E, Detterbeck F, Van Raemdonck D, et al. Tumours of the thymus: A cohort study of prognostic factors from the European Society of Thoracic Surgeons database. Eur J Cardiothorac Surg 2014;46:361-8. [Crossref] [PubMed]
- Regnard JF, Magdeleinat P, Dromer C, et al. Prognostic factors and long-term results after thymoma resection: A series of 307 patients. J Thorac Cardiovasc Surg 1996;112:376-84. [Crossref] [PubMed]
- Kondo K. Optimal therapy for thymoma. J Med Invest 2008;55:17-28. [Crossref] [PubMed]
- Basse C, Thureau S, Bota S, et al. Multidisciplinary Tumor Board Decision Making for Postoperative Radiotherapy in Thymic Epithelial Tumors: Insights from the RYTHMIC Prospective Cohort. J Thorac Oncol 2017;12:1715-22. [Crossref] [PubMed]
- Sugie C, Shibamoto Y, Ikeya-Hashizume C, et al. Invasive thymoma: Postoperative mediastinal irradiation, and low-dose entire hemithorax irradiation in patients with pleural dissemination. J Thorac Oncol 2008;3:75-81. [Crossref] [PubMed]
- Masaoka A. Staging system of thymoma. J Thorac Oncol 2010;5:S304-12. [Crossref] [PubMed]
- Ruffini E, Mancuso M, Oliaro A, et al. Recurrence of thymoma: analysis of clinicopathologic features, treatment, and outcome. J Thorac Cardiovasc Surg 1997;113:55-63. [Crossref] [PubMed]
- Kim YJ, Kim SS, Song SY, et al. Elective Nodal Irradiation as Adjuvant Radiotherapy for Advanced Thymomas and Thymic Carcinomas. Clin Lung Cancer 2019;20:e91-e96. [Crossref] [PubMed]
- Detterbeck FC. Clinical Value of the WHO Classification System of Thymoma. Ann Thorac Surg 2006;81:2328-34. [Crossref] [PubMed]
- Patel S, MacDonald OK, Nagda S, et al. Evaluation of the role of radiation therapy in the management of malignant thymoma. Int J Radiat Oncol Biol Phys 2012;82:1797-801. [Crossref] [PubMed]
- Omasa M, Date H, Sozu T, et al. Postoperative radiotherapy is effective for thymic carcinoma but not for thymoma in stage II and III thymic epithelial tumors: The Japanese Association for Research on the Thymus Database Study. Cancer 2015;121:1008-16. [Crossref] [PubMed]
- Fernandes AT, Shinohara ET, Guo M, et al. The role of radiation therapy in malignant thymoma: A surveillance, epidemiology, and end results database analysis. J Thorac Oncol 2010;5:1454-60. [Crossref] [PubMed]
- Forquer JA, Rong N, Fakiris AJ, et al. Postoperative radiotherapy after surgical resection of thymoma: differing roles in localized and regional disease. Int J Radiat Oncol Biol Phys 2010;76:440-5. [Crossref] [PubMed]
- Zhang H, Lu N, Wang M, et al. Postoperative radiotherapy for stage I thymoma: A prospective randomized trial in 29 cases. Chin Med J (Engl) 1999;112:136-8. [PubMed]
- Liu Q, Gu Z, Yang F, et al. Role of postoperative radiotherapy for stage I/II/III thymic tumor - Results of the ChART retrospective database. Zhongguo Fei Ai Za Zhi 2016;19:465-72. [Crossref] [PubMed]
- Jackson MW, Palma DA, Camidge DR, et al. The Impact of Postoperative Radiotherapy for Thymoma and Thymic Carcinoma. J Thorac Oncol 2017;12:734-44. [Crossref] [PubMed]
- Zhou D, Deng XF, Liu QX, et al. The effectiveness of postoperative radiotherapy in patients with completely resected thymoma: A meta-analysis. Ann Thorac Surg 2016;101:305-10. [Crossref] [PubMed]
- Ma J, Sun X, Huang L, et al. Postoperative radiotherapy and tumor recurrence after complete resection of stage II /III thymic tumor: A meta-analysis of cohort studies. Onco Targets Ther 2016;9:4517-26. [Crossref] [PubMed]
- Rimner A, Yao X, Huang J, et al. Postoperative radiation therapy is associated with longer overall survival in completely resected stage II and III thymoma-an analysis of the international thymic malignancies interest group retrospective database. J Thorac Oncol 2016;11:1785-92. [Crossref] [PubMed]
- Liao J, Liu T, Zhang H, et al. The role of postoperative radiation therapy for completely resected stage III thymoma and effect of higher heart radiation dose on risk of cardiovascular disease: A retrospective cohort study. Int J Surg 2018;53:345-9. [Crossref] [PubMed]
- Mou H, Liao Q, Hou X, et al. Clinical characteristics, risk factors, and outcomes after adjuvant radiotherapy for patients with thymoma in the United States: analysis of the Surveillance, Epidemiology, and End Results (SEER) Registry (1988–2013). Int J Radiat Biol 2018;94:495-502. [Crossref] [PubMed]
- Weksler B, Shende M, Nason KS, et al. The role of adjuvant radiation therapy for resected stage III thymoma: A population-based study. Ann Thorac Surg 2012;93:1822-8. [Crossref] [PubMed]
- Yan J, Liu Q, Moseley JN, et al. Adjuvant radiotherapy for stages II and III resected thymoma: A single-institutional experience. Am J Clin Oncol 2016;39:223-7. [Crossref] [PubMed]
- Boothe D, Orton A, Thorpe C, et al. Postoperative radiotherapy in locally invasive malignancies of the thymus: patterns of care and survival. J Thorac Oncol 2016;11:2218-26. [Crossref] [PubMed]
- Lim YJ, Kim HJ, Wu HG. Role of Postoperative radiotherapy in nonlocalized thymoma: propensity-matched analysis of Surveillance, Epidemiology, and End Results Database. J Thorac Oncol 2015;10:1357-63. [Crossref] [PubMed]