
Feasibility and accuracy of rapid on-site evaluation of touch imprint cytology during transbronchial biopsy
Introduction
Rapid on-site evaluation (ROSE) during bronchoscopy has a great importance, because it provides instant feedback on whether the obtained specimens include the target lesions. Although several reports have described the utility of ROSE, most of these reports focused on ROSE during transbronchial needle aspiration (TBNA). The first randomized trial evaluating the usefulness of ROSE during conventional TBNA was conducted by Trisolini et al. in patients with lymphadenopathy. They concluded that ROSE can help avoid additional biopsies without a loss in diagnostic yield (1). Oki et al. published the first randomized trial aimed at assessing the utility of ROSE during endobronchial ultrasound-guided TBNA (EBUS-TBNA) for the diagnosis of lymph node metastasis in lung cancer. Patients with ROSE during EBUS-TBNA were significantly more likely to avoid additional bronchoscopic procedures (2). However, there have been only a few reports on ROSE during transbronchial biopsy (TBB) for peripheral pulmonary lesions (PPLs). TBB is widely performed for the diagnostic evaluation of patients with lung cancer or other lung diseases because of less complications, compared with those of CT-guided transthoracic biopsy (3,4). Sufficient tissue materials are required for defining the subtypes of lung cancer, testing gene mutations, and analyzing PD-L1 expression, all of which are essential for targeted therapy or precision medicine (5); TBB has been reported to be sufficient for such evaluations (6,7).
Touch imprint cytology (TIC) is a simple and rapid technique and was reported to be an effective cytological assessment on TBB specimens (8,9). Now, as therapy for lung cancer has dramatically changed, clinical requirements for bronchoscopic sampling are also changing; sufficient numbers of specimens of high quality are clinically required. Regarding conducting TBB, radial probe EBUS has been developed for better diagnostic yield for PPLs (10,11). There are few evidences on the utility of ROSE-TIC during TBB in the era of “advanced” techniques and therapies. Thus, this study aims to assess the feasibility and accuracy of ROSE-TIC by showing the correlation among the results of ROSE-TIC, histological findings, and final diagnosis and show the success rate of molecular testing for targeted therapy using bronchoscopic specimens.
Methods
Study design and patients
This was a single-center retrospective chart review of 528 patients who underwent bronchoscopy combined with ROSE at the Chiba University Hospital between January 2014 and September 2017. All analyses were performed in accordance with the amended Declaration of Helsinki. Written informed consent for bronchoscopy was obtained from each patient. Because data anonymization and privacy issues were protected and the approval for the opt-out consent method were given by the Chiba University Hospital (approval number 2584), additional informed consent for this research was waived due to the design of retrospective chart review of clinical history and diagnostic results.
Bronchoscopy
All examinations were performed using a flexible bronchoscope. The bronchoscope was inserted through the oral route under mild sedation following pharyngeal anesthesia. In cases of PPLs, virtual bronchoscopic navigation (Ziostation2; AMIN, Japan) was created prior to performing endobronchial ultrasound with a guide sheath (EBUS-GS). The radial EBUS probe (20 MHz mechanical radial type, UM-S20-20R or UM-S20-17S; Olympus, Japan) was inserted into the GS kit (K-201 or K-203; Olympus, Japan). After reaching the target lesion, TBB, brushing, and/or needle aspiration was performed under fluoroscopic guidance. In this study, bronchoscopically visible target lesions were defined as central lesions, whereas other lesions were classified as peripheral lesions.
Specimen handling
The obtained materials from forceps biopsy were touched onto glass slides as imprint cytology. The glass slides were air-dried, stained by Diff-Quik stain (American Scientific Products, McGaw Park, IL). Pictures of the procedure are shown in Figure S1. The remaining materials were placed in 10% neutral buffered formalin and were then embedded in paraffin for histological evaluation on hematoxylin and eosin staining. The cells sampled by brushing or needle aspiration were spread onto two glass slides; one slide was fixed in 95% ethanol for Papanicolaou staining, and the other slide was air-dried for ROSE for brushing cytology (ROSE-BC) and ROSE for transbronchial needle aspiration cytology (ROSE-AC).
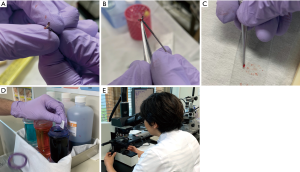
ROSE and final diagnosis
ROSE was performed by a cytotechnologist who was certified by the Japanese Society of Clinical Cytology. The materials were diagnosed and categorized as either positive or negative for malignancy. The term “positive” was defined as the presence of cancer cells or cells suspicious for malignancy. The results of ROSE-TIC were compared with the histological findings and final diagnosis. The results of the ROSE-BC or ROSE-AC were compared with the cytological findings and final diagnosis. For the cytological assessment, malignant cells were defined as class III to class V according to the Papanicolaou classification. Final diagnosis was defined as the outcome of the histological findings on bronchoscopy or other procedures, such as CT-guided transthoracic needle aspiration or surgical resection, observation, and response to medical treatments.
Molecular testing
Molecular testing was performed in patients diagnosed with non-small cell lung cancer (NSCLC). EGFR mutation status was evaluated by the peptide nucleic acid-locked nucleic acid polymerase chain reaction (PCR) clamp method. To detect anaplastic lymphoma receptor tyrosine kinase ALK rearrangements, immunohistochemistry scoring and fluorescent in situ hybridization were performed. Reverse transcriptase PCR was performed to detect ROS1 rearrangements. These evaluations were conducted by an outsourcing company (LSI Medience Corp., Tokyo, Japan). PD-L1 expression was analyzed in-house using the PD-L1 IHC Dako 22C3 pharmDx assay (Dako autostainer Link 48).
Statistical analysis
The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy of ROSE-TIC were calculated according to standard definitions. The success rates of ROSE-TIC for molecular analysis were also calculated. Data analysis was performed using Microsoft Excel software package (Microsoft Corporation, Redmond, WA, USA).
Results
Study population and characteristics
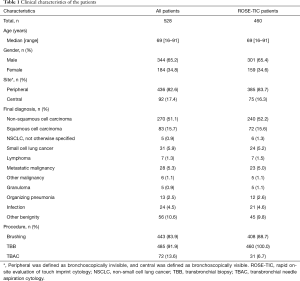
In total, 528 patients underwent bronchoscopy with ROSE, in which 460 patients underwent bronchoscopy with ROSE-TIC. Table 1 summarizes the patient characteristics. There were 301 males (65.4%) and 159 females (34.6%), and the median age was 69 (range, 16–91) years. Diagnostic bronchoscopy was performed in 385 cases (83.7%) for PPLs and 75 cases (16.3%) for central lesions. The final diagnoses comprised 377 malignant cases (82.0%) and 83 non-malignant cases (18.0%). A total of 99 cases (21.5%) were not diagnosed by bronchoscopy. Of these, diagnosis was achieved by observation or in response to medical treatment in 41 cases (41.0%), by surgical resection in 39 cases (39.0%), by performing an additional bronchoscopy in 13 cases (13.0%), and by CT-guided transthoracic needle aspiration in 2 cases (2.0%). In cases diagnosed by observation or in response to medical treatment, the mean time from performing bronchoscopy to reaching the diagnosis was 6.6 months (Tables S1,S2).
Full table

Full table

Full table
Correlation between ROSE and examination results
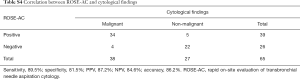
First, compared with the histological findings, ROSE-TIC showed sensitivity, specificity, PPV, NPV, and diagnostic accuracy values of 91.1%, 90.4%, 94.8%, 84.0%, and 90.9%, respectively and discordant results were shown in 42 cases (9.1%) (Table 2). Second, compared with the final diagnosis, ROSE-TIC showed sensitivity specificity, PPV, NPV, and diagnostic accuracy values of 75.3%, 91.6%, 97.6%, 45.0%, and 78.3%, respectively, and discordant results were shown in 100 cases (21.7%) (Table 3). The same calculations were performed for ROSE-BC and ROSE-AC, as shown in Tables S3-S6.

Full table

Full table

Full table
Full table

Full table

Full table
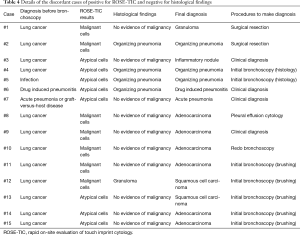
Among the 42 cases in which the ROSE-TIC results and the histological findings were discordant, 15 cases (3.3%) were positive according to ROSE-TIC and negative for the histological findings. The details are summarized in Table 4. Seven of the 15 cases (1.5%) were negative at the final diagnosis, that is, they were false-positive cases, and 4 of those cases were suspicious for lung cancer before bronchoscopy and were finally diagnosed with interstitial lung disease. Eight of the 15 cases (1.7%) were positive at the final diagnosis. Four cases were positive according to brushing cytology during the same bronchoscopy; thus, those patients did not receive further examinations for determining a diagnosis.
Full table
Evaluation of molecular analysis
As shown in Table 5, the success rates of ROSE-TIC for molecular analysis testing of the NSCLC cases were 96.6% for EGFR mutation, 87.3% for ALK rearrangement, 93.1% for ROS1 rearrangement, and 96.2% for PD-L1 expression.

Full table
Discussion
In this study, we demonstrated two important features in clinical practice. First, ROSE-TIC showed high correlation with the histologic findings and final diagnosis with high sensitivity and PPV. Second, tissue sampling by TBB combined with ROSE-TIC showed high success rate for molecular testing for targeted therapy. These results suggest the clinical feasibility and accuracy of ROSE-TIC during TBB.
Our results showed that the results of ROSE-TIC were highly correlated with the histological findings and the final diagnosis. A previous study for EBUS-GS cases showed that ROSE-TIC for PPLs had a high accuracy, even in cases with ground-glass opacities (12). Another recent published report demonstrated that although ROSE-TIC for TBB did not improve the overall diagnostic yield, it improved the accuracy for PPLs (13). ROSE for malignant cases had a high concordance rate with the histological findings and that it may be useful for early clinical decision-making. A positive result on ROSE provides useful information to bronchoscopists, guiding the decision on finishing procedures after sampling enough tissues. This way, the number of biopsies may be reduced, and the procedure time may be shortened, as previously reported (2,14). Moreover, the invasiveness of the procedure may be minimized, and treatment can be administered sooner. On the other hand, the proportion of discordant cases that were positive on ROSE-TIC and negative on histological findings was 3.3% (15 cases) and higher than those reported in previous studies (12,13). Moreover, as shown in Table 3, the proportion of discordant cases that were positive on ROSE-TIC but negative on final diagnosis was 1.5% (7 cases). Notably, only few studies have compared the results of ROSE and the final results. One of the reasons for the discrepancy in the results between our study and the previous studies could be our categorization of atypical cells as positive for malignancy. Many cases of our discordant cases were diagnosed as atypical cells, which may have reflected reactive changes secondary to inflammation and not necessarily be suspicious for malignancy. However, in case 1, the ROSE-TIC results were suggestive of adenocarcinoma but required further histological examination for confirmation of adenocarcinoma (Figure S2). A previous report pointed out that the diagnosis of PPLs is more difficult than that of lymph nodes due to the presence of bronchial ciliated epithelium, bronchial cartilage, and abundant inflammatory cells around the lesion (12). Another report suggested that marked reactive atypia and metaplastic changes in the bronchial epithelium and dysplasia of squamous cells potentially cause false-positive diagnoses (15). Moreover, because the diagnosis by ROSE-TIC needs to be immediate and is based on a small amount of information, complete examination of the specimen for diagnosing benignancy or malignancy may be difficult. For cases 1 and 2 in this study, the cytotechnologist has received clinical information on the suspicion for malignancy in advance from the physician; this may have influenced the ROSE-TIC reading of malignancy. Another factor to consider was the difference between the person who read the ROSE-TIC and the one who evaluated the histopathology. Therefore, false positives on ROSE-TIC should be always considered until the final histological results are acquired. Important clinical decisions that may directly affect patient outcomes such as implementing chemotherapy should not be performed based on the ROSE results alone. Although paying attention to its interpretation is necessary, ROSE-TIC had a sufficiently high reliability, considering the high sensitivity and PPV shown in our results.
Most of the tissue samples on which we performed ROSE-TIC were evaluable for molecular analysis and PD-L1 expression. The evidence-based guidelines published by the College of American Pathologists suggested that EGFR, ALK, and ROS1 testing should be performed for molecular therapy (16). Some studies reported that PD-L1 expression should be assessed for immunotherapy and can be a predictor of better survival in patients treated with immune checkpoint inhibitors (16,17). Because analyses of molecular mutations and PD-L1 expression mainly necessitate the use of tissue specimens, repeated biopsies are required (18). In this study, these evaluations were highly successful as shown in Table 5. As mentioned above, ROSE-TIC showed high sensitivity and PPV; therefore, positive ROSE-TIC results confirm the adequate location for sampling. Procurement of sufficient amount of sufficient materials can enable the evaluation of molecular mutations and PD-L1 expression for targeted therapy.
There were several limitations of our study. First, this was a retrospective and single-center study. Although ROSE-TIC showed high concordance with the histological and final results, its value in improving the diagnostic yield was not certain. Second, all ROSE-TIC procedures were performed only by one cytotechnologist. ROSE-TIC should be performed by multiple cytotechnologists to realize universality. A prospective, randomized, multicenter study is needed to confirm these matters.
In conclusion, ROSE-TIC showed high correlation with the histologic findings and final diagnosis. Moreover, tissue sampling combined with ROSE-TIC resulted in high success rate for molecular analysis for targeted therapy; however, further study is needed to validate our results.
Acknowledgments
The authors would like to thank Enago for the English language review.
Funding: This work was supported in part by funding from the Intractable Respiratory Diseases and Pulmonary Hypertension Research Group from the Ministry of Health, Labor and Welfare of Japan.
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-671
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-671). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures involving human participants were approved by the Human Ethics Committee of the Graduate School of Medicine of Chiba University (approval number 2584). The participants had the option to opt out from the study. The requirement for informed consent was waived by the ethics committee, because this retrospective analysis was limited to preexisting data that were collected as part of the standard of care by respiratory physicians, and data anonymization and privacy issues were protected.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Trisolini R, Cancellieri A, Tinelli C, et al. Rapid on-site evaluation of transbronchial aspirates in the diagnosis of hilar and mediastinal adenopathy: a randomized trial. Chest 2011;139:395-401. [Crossref] [PubMed]
- Oki M, Saka H, Kitagawa C, et al. Rapid on-site cytologic evaluation during endobronchial ultrasound-guided transbronchial needle aspiration for diagnosing lung cancer: a randomized study. Respiration 2013;85:486-92. [Crossref] [PubMed]
- Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology 2013;266:304-17. [Crossref] [PubMed]
- Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e93S-120S.
- Folch E, Costa DB, Wright J, et al. Lung cancer diagnosis and staging in the minimally invasive age with increasing demands for tissue analysis. Transl Lung Cancer Res 2015;4:392-403. [PubMed]
- Kage H, Kohsaka S, Shinozaki-Ushiku A, et al. Small lung tumor biopsy samples are feasible for high quality targeted next generation sequencing. Cancer Sci 2019;110:2652-57. [PubMed]
- Tsunoda A, Morikawa K, Inoue T, et al. A prospective observational study to assess PD-L1 expression in small biopsy samples for non-small-cell lung cancer. BMC Cancer 2019;19:546. [Crossref] [PubMed]
- Popp W, Rauscher H, Ritschka L, et al. Diagnostic sensitivity of different techniques in the diagnosis of lung tumors with the flexible fiberoptic bronchoscope. Comparison of brush biopsy, imprint cytology of forceps biopsy, and histology of forceps biopsy. Cancer 1991;67:72-5. [Crossref] [PubMed]
- Kawaraya M, Gemba K, Ueoka H, et al. Evaluation of various cytological examinations by bronchoscopy in the diagnosis of peripheral lung cancer. Br J Cancer 2003;89:1885-8. [Crossref] [PubMed]
- Paone G, Nicastri E, Lucantoni G, et al. Endobronchial ultrasound-driven biopsy in the diagnosis of peripheral lung lesions. Chest 2005;128:3551-7. [Crossref] [PubMed]
- Herth FJ, Eberhardt R, Becker HD, et al. Endobronchial ultrasound-guided transbronchial lung biopsy in fluoroscopically invisible solitary pulmonary nodules: a prospective trial. Chest 2006;129:147-50. [Crossref] [PubMed]
- Izumo T, Matsumoto Y, Sasada S, et al. Utility of rapid on-site cytologic evaluation during endobronchial ultrasound with a guide sheath for peripheral pulmonary lesions. Jpn J Clin Oncol 2017;47:221-5. [Crossref] [PubMed]
- Wang J, Zhao Y, Chen Q, et al. Diagnostic value of rapid on-site evaluation during transbronchial biopsy for peripheral lung cancer. Jpn J Clin Oncol 2019;49:501-5. [Crossref] [PubMed]
- Diacon AH, Schuurmans MM, Theron J, et al. Utility of rapid on-site evaluation of transbronchial needle aspirates. Respiration 2005;72:182-8. [Crossref] [PubMed]
- Alsharif M, Andrade RS, Groth SS, et al. Endobronchial ultrasound-guided transbronchial fine-needle aspiration: the University of Minnesota experience, with emphasis on usefulness, adequacy assessment, and diagnostic difficulties. Am J Clin Pathol 2008;130:434-43. [Crossref] [PubMed]
- Lindeman NI, Cagle PT, Aisner DL, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Mol Diagn 2018;20:129-59. [Crossref] [PubMed]
- Aguiar PN Jr, De Mello RA, Hall P, et al. PD-L1 expression as a predictive biomarker in advanced non-small-cell lung cancer: updated survival data. Immunotherapy 2017;9:499-506. [Crossref] [PubMed]
- Brown NA, Aisner DL, Oxnard GR. Precision medicine in non-small cell lung cancer: current standards in pathology and biomarker interpretation. Am Soc Clin Oncol Educ Book 2018;38:708-15. [Crossref] [PubMed]


