|
Case Report
Successful treatment of gefitinib-induced acute interstitial
pneumonitis with corticosteroid and non-invasive BIPAP-ventilation
Yalei Zhang, Haihong Yang, Meiling Zhao, Jianxing He
Department of Cardiothoracic Surgery, The First Affiliated Hospital of Guangzhou Medical College, No. 151, Yanjiang Rd, Guangzhou,
510120, China
Corresponding to: Jianxing He. Department of Cardiothoracic Surgery, The First
Affiliated Hospital of Guangzhou Medical College, No. 151, Yanjiang Rd, Guangzhou,
510120, China. Tel: 86-20-83337792; Fax:86-20-83350363. Email: hejx@vip.163.
com.
|
|
Abstract
This is the case of a 63 year-old male who was diagnosed adenocarcinoma in the left upper lung with ipsilateral malignant
pleural effusion. At diagnosis it had already spread to left pulmonary HLN (hilar lymph node) and left supraclavicular
lymph node and mediastinal lymph nodes. The patient received combined chemotherapy with bevacizumab and GP
(gemcitabine and carboplatin) for 6 courses. Disease progression on chest CT scan was recognized, daily treatment with
oral gefitinib (250 mg/day) was commenced. One week later, he was admitted under the impression of gefitinib-related
interstitial pneumonitis, gefitinib was discontinued immediately and methylprednisolone with BIPAP assisted ventilation
were used. The patient was followed up for 2 months after the start of treatment with corticosteroids and BIPAP assisted
ventilation and remained well.
Key words
Gefitinib; interstitial pneumonitis; non-invasive BIPAP-ventilation
J Thorac Dis 2012;4(3):316-319. DOI: 10.3978/j.issn.2072-1439.2012.03.20 |
|
Introduction
Lung cancer remains the leading cause of malignancy-related
mortality worldwide, with over one million cases diagnosed
yearly ( 1). Non-small-cell lung cancer (NSCLC) accounts for
>80% of all lung cancers. Because it is typically diagnosed at an
advanced stage, chemotherapy (CT) remains the cornerstone
of treatment, however, conventional treatment of NSCLC
has apparently reached a plateau of effectiveness in improving
survival of patients. For better toxicity profile than conventional
CT, molecular targeted therapies for cancer become a new
therapeutic areas in recent years. Gefitinib (Iressa) is an
Epidermal Growth Factor Receptor Type 1/tyrosine kinase
(HER1/EGFR) inhibitor and block the signal transduction
pathway implicated in the proliferation and survival of cancer
cells ( 2, 3). The development of gefitinib in the treatment of advanced non-small-cell-lung cancer (NSCLC) raised a great
enthusiasm among physicians. Gefitinib is well tolerated and less
toxic compared to conventional cytotoxic drugs, but gefitinibrelated
interstitial lung disease (ILD) has been reported as a
serious adverse effect ( 4). Here, we reported a case of acute lung
injury induced by gefitinib that was detected in the early phase
with high-resolution (HR) CT, and successfully treated with
corticosteroid and BIPAP assisted ventilation therapy. |
|
Case report
A 63-year-old man presented with cough and dyspnoea on
exertion. He had been previously healthy until adenocarcinoma
in the left upper lung with ipsilateral malignant pleural
effusion and left pulmonary HLN (hilar lymph node) and
left supraclavicular lymph node and mediastinal lymph nodes
metastasis (T1N3M1a, stage IV) was diagnosed in May 2010.
He received combined chemotherapy with bevacizumab
(Avastin) 400 mg d0/3 weeks and gemcitabine 1,600 mg d1, 8/3
weeks and carboplatin 500 mg d1/3weeks for 6 courses. At the
same time, left-sided intrathoracic instillation of OK-432 was
given. Disease progression on chest CT scan was recognized in
December 2010. Daily treatment with oral gefitinib (250 mg/
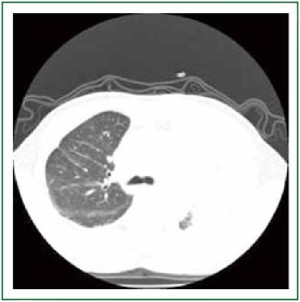
day) was commenced. At this time his CT scan showed no lung
tissue abnormality in the right upper lobe (Figure 1). The patient
then received gefitinib (250 mg/day) on December 20, 2010.
However, aggravated dyspnea on exertion with a dry cough developed after 7 days of gefitinib treatment. Hypoxemia and
a 75% of oxyhemoglobin saturation at rest were measured by
pulse oximetry. The body temperature was 37.0 ℃; pulse rate
was 100 beats/min and the blood pressure was 135/85 mmHg.
Arterial blood gas analysis at rest (FiO2 =0.29) were illustrated in
Table 1 (Values 1). Leucocyte cell count was 1.0×104/mm3 with
77% neutrophils. All cultures and stains for infectious etiologies
including common bacteria, fungi, pneumocystis, legionella,
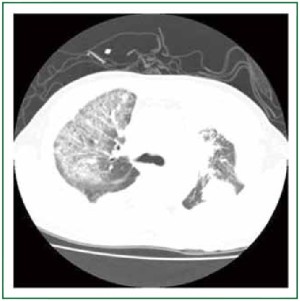
nocardia, viruses were negative. Chest CT revealed ground
glass opacity (GGO) in the right upper lobe (Figure 2), which
suggesting an acute interstitial pneumonia. He was admitted
under the impression of gefitinib-related interstitial pneumonitis.
After admission, gefitinib was discontinued immediately and
methylprednisolone 80 mg/day was started. At the same time,
BIPAP assisted ventilation was used. The Ventilator parameters
were: IPAP 12 cmH2O, EPAP 4 cmH2O, FiO2 75%, R 18 bpm.
Dyspnoea and cough were resolved 7 days after commencement
of treatment with methylprednisolone and BIPAP assisted
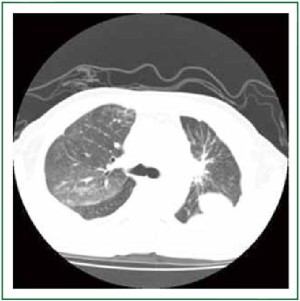
ventilation, and a chest CT taken 10 days after the start of
treatments demonstrated a scar-like lesion without GGO in the right upper lobe (Figure 3). Arterial blood gas analysis in
room air (FiO2 =0.29) were illustrated in Table 1 (Values 2). The
daily dosage of prednisolone was decreased by 20 mg per day
depending on the patient’s response. The patient was followed
up for 2 months after the start of treatment with corticosteroids
and remained well.
|
|
Discussion
Gefitinib is an oral selective inhibitor of the epidermal growth
factor receptor (EGFR) tyrosine kinase that may be effective in
some patients with advanced non-small-cell lung, ovarian, breast,
head and neck, and colon cancers. Members of the EGF family
have been implicated in the repair of pulmonary damage ( 5, 6).
Therefore, inhibition of EGFR-mediated signalling by gefitinib
may impair the repair of bronchioloalveolar epithelium and
thereby exacerbate lung injury ( 7, 8), especially in patients with
pulmonary comorbidities ( 9). Although the incidence of lung injury due to gefitinib is low
( 10), the number of patients with gefitinib-induced lung injury
is likely to increase. Therefore, a strategy for dealing with this is
required. There was no randomized controlled trial to guide the
management of gefitinib-induced acute interstitial pneumonia.
Li-Chiao Kuo et al. ( 11) used high-dose corticosteroid to treat
gefitinib-induced acute interstitial pneumonia,but high-dose
corticosteroid is harmful to the body. The case in the present
report suggests that lung injury induced by gefitinib could be
treated and cured using low-dose corticosteroid and BIPAP
assisted ventilation, if detected early. When dyspnoea on
exertion developed in this patient 1 week after administration
of oral gefitinib, CT was immediately performed and gefitinib
discontinued. At that time, this patient was serious, we can’t carry
out the transbronchial biopsy. Ten days after discontinuation,
HRCT showed progression of lung injury, which was compatible
with an AIP pattern. Treatment with corticosteroids and BIPAP
assisted ventilation resolved the lung injury, symptoms and the
GGO lesions on HRCT, and increased PaO 2. Such recovery is
possible when lung injury is detected and treated in the early
phase of diffuse alveolar damage, before progression to the
fibrotic phase of AIP. Noninvasive ventilation (NIV) has revolutionised the ( 12)
management of patients with acute respiratory failure. It has
decreased the need for endotracheal intubation and its attendant
complications like nosocomial pneumonia and ( 13) other
intensive care unit-acquired infections. In selected situations like
chronic obstructive pulmonary disease and pulmonary oedema,
it has also been shown to decrease ( 14, 15) mortality. Positive
pressure therapy acts by augmenting ( 16, 17) intrinsic positive
end-expiratory pressure (PEEP). Using biphasic positive airway
pressure (BiPAP), may improve the respiratory failure in patients
with type II blood oxygen level, to improve blood oxygen
pressure, oxygen saturation and improved tissue hypoxia. To effectively follow up patients treated with gefitinib and
to prevent deaths from gefitinib-induced lung injury, early
recognition and intervention may prevent a fatal outcome,
corticosteroids and non-invasive BIPAP-ventilation should be
considered if clinically deteriorated especially the patient has
severe hypoxia.
|
|
Acknowledgements
Disclosure: The authors declare no conflict of interest.
|
|
References
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics,
2008. CA Cancer J Clin 2008;58:71-96.
- Arteaga CL, Johnson DH. Tyrosine kinase inhibitors-ZD1839 (Iressa).
Curr Opin Oncol 2001;13:491-8.
- Ciardiello F, Tortora G. A novel approach in the treatment of cancer:
targeting the epidermal growth factor receptor. Clin Cancer Res
2001;7:2958-70.
- Inoue A, Saijo Y, Maemondo M, Gomi K, Tokue Y, Kimura Y, et al. Severe
acute interstitial pneumonia and gefitinib. Lancet 2003;361:137-9.
- Madtes DK, Busby HK, Strandjord TP, Clark JG. Expression of
transforming growth factor-alpha and epidermal growth factor receptor is
increased following bleomycin-induced lung injury in rats. Am J Respir Cell
Mol Biol 1994;11:540-51.
- Madtes DK, Rubenfeld G, Klima LD, Milberg JA, Steinberg KP, Martin TR,
et al. Elevated transforming growth factor-alpha levels in bronchoalveolar
lavage fluid of patients with acute respiratory distress syndrome. Am J
Respir Crit Care Med 1998;158:424-30.
- Hardie WD, Prows DR, Leikauf GD, Korfhagen TR. Attenuation of acute
lung injury in transgenic mice expressing human transforming growth
factor-alpha. Am J Physiol 1999;277:L1045-50.
- Hardie WD, Prows DR, Piljan-Gentle A, Dunlavy MR, Wesselkamper SC,
Leikauf GD, et al. Dose-related protection from nickel-induced lung injury
in transgenic mice expressing human transforming growth factor-alpha. Am
J Respir Cell Mol Biol 2002;26:430-7.
- Danson S, Blackhall F, Hulse P, Ranson M. Interstitial lung disease in lung
cancer: separating disease progression from treatment effects. Drug Saf
2005;28:103-13.
- Armour A.Gefitinib in advanced non-small cell lung cancer:clinical
experience in patients of Asian origin. Asia Pac J Clin Oncol 2007;3:366-78.
- Kuo LC, Lin PC, Wang KF, Yuan MK, Chang SC. Successful treatment of
gefitinib-induced acute interstitial pneumonitis with high-dose corticosteroid:
a case report and literature review. Med Oncol 2011;28:79-82.
- Brochard L. Noninvasive ventilation for acute respiratory failure. JAMA
2002;288:932-5.
- Girou E, Brun-Buisson C, Taillé S, Lemaire F, Brochard L. Secular trends
in nosocomial infections and mortality associated with noninvasive
ventilation in patients with exacerbation of COPD and pulmonary edema. JAMA 2003;290:2985-91.
- Ram FS, Picot J, Lightowler J, Wedzicha JA. Non-invasive positive pressure
ventilation for treatment of respiratory failure due to exacerbations of
chronic obstructive pulmonary disease. Cochrane Database Syst Rev
2004;CD004104.
- Agarwal R, Aggarwal AN, Gupta D, Jindal SK. Non-invasive ventilation in
acute cardiogenic pulmonary oedema. Postgrad Med J 2005;81:637-43.
- Katz JA, Kraemer RW, Gjerde GE. Inspiratory work and airway pressure
with continuous positive airway pressure delivery systems. Chest
1985;88:519-26.
- Lenique F, Habis M, Lofaso F, Dubois-Randé JL, Harf A, Brochard L.
Ventilatory and hemodynamic effects of continuous positive airway
pressure in left heart failure. Am J Respir Crit Care Med 1997;155:500-5.
Cite this article as: Zhang Y, Yang H, Zhao M, He J. Successful treatment of
gefitinib-induced acute interstitial pneumonitis with corticosteroid and Noninvasive
BIPAP-ventilation: A case report. J Thorac Dis 2012;4(3):316-319.
doi: 10.3978/j.issn.2072-1439.2012.03.20
|