
The clinical efficacy of lefamulin in the treatment of elderly patients with community-acquired bacterial pneumonia
Community-acquired bacterial pneumonia (CABP) remains a common cause of elderly patients requiring hospitalization and it can also be associated with significant morbidity and mortality (1-3). Common causative pathogens of CAPB include typical pathogens—Streptococcus pneumoniae, Staphylococcus aureus, Haemophilus influenzae, Moraxella catarrhalis, and the atypical pathogens—Mycoplasma pneumoniae, Chlamydophila pneumoniae, and Legionella pneumophila. Appropriate antibiotics are the key of successful management of elderly patients with CABP (2), however, the emergence of antimicrobial resistance among these respiratory pathogens limits the choice of antibiotic and could negatively impact the outcomes of the elderly patients with CAPB.
Fortunately, recent development of a novel antibiotic—lefamulin, the first systemic pleuromutilin antibiotic, is active against the most common CABP-causing pathogens, including both typical and atypical pathogens and can provide us a therapeutic alternative in this clinical entity (4). In addition to in vitro studies (5,6), two phase III studies—Lefamulin Evaluation Against Pneumonia (LEAP) 1 trial (7) and LEAP 2 trial (8) have reported the clinical efficacy and safety profile of lefamulin for treating CABP. However, the usefulness of lefamulin in the elderly patients remains unclear. Therefore, we extracted the results of LEAP 1 and 2 trials (7,8) for the elderly patients and did a meta-analysis to investigate the clinical efficacy of lefamulin in the treatment of elderly patients with CABP.
Both LEAP 1 and 2 trials were phase III, randomized, double-blind, double-dummy, multicenter, multinational studies (7,8). LEAP 1 trial (7) compared the usefulness of iv-to-oral lefamulin and moxifloxacin ± linezolid in the treatment of adult patients with CABP at Pneumonia Outcomes Research Team (PORT) risk class ≥ III. LEAP 2 trial compared the clinical efficacy of oral lefamulin and oral moxifloxacin for treating the adult patients with CABP at PORT risk class II, III or IV (8). The primary outcomes included early clinical response at 96±24 hours after first study drug dose in the intent-to-treat (ITT) population and investigator assessment of clinical response at test of cure (TOC) (5–10 days after end of treatment) in the modified ITT population. The ITT population included all randomized patients and the modified ITT population included randomized patients who received any amount of study drug.
Overall, a total of 517 elderly patients aged ≥65 years were included in this analysis. Two hundred and sixty-eight and two hundred forty-nine elderly patients with CABP were randomized to receive lefamulin and moxifloxacin, respectively. The early clinical response rate was 91.0% (244/268) and 86.7% (216/249) among lefamulin and moxifloxacin group, respectively. No significant difference was observed between lefamulin and moxifloxacin [odds ratio (OR), 1.58; 95% confidence interval (CI), 0.90–2.75, I2=0%] (Figure 1). The clinical response rate at TOC was 88.8% (237/267) and 83.5% (207/248) among lefamulin and moxifloxacin (OR, 1.61; 95% CI, 0.97–2.67, I2=0%) (Figure 1).
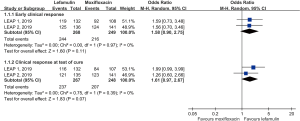
In the subgroup analysis, no significant differences in terms of early clinical response rate was found between lefamulin and moxifloxacin for patients aged 65–74 years (OR, 2.12; 95% CI, 0.94–4.78, I2=0) and aged ≥75 years (OR, 1.18; 95% CI, 0.53–2.61, I2=0). Regarding the clinical responses rate at TOC, the similar trend was observed both subgroups (65–74 years, OR, 1.66; 95% CI, 0.77–3.56, I2=24%; ≥75 years, OR, 1.53; 95% CI, 0.69–3.38, I2=0%).
Based on this integrated analysis of LEAP 1 and LEAP 2 trials, we demonstrated that the clinical efficacy of lefamulin in the treatment of elderly patients with CABP was similar to moxifloxacin. The similarity of lefamulin to moxifloxacin was demonstrated in overall elderly population in different timing of outcome measurement—early clinical response at 96±24 hours after first study drug dose and clinical response at TOC and also across the different age populations—aged 65–74 years and aged ≥75 years. In addition, the outcomes of elderly patients with CABP undergoing lefamulin treatment achieved to the clinical response rate of more than 88%. All of these findings indicated that lefamulin exhibited good efficacy in the treatment of elderly patients with CABP and should suggest that lefamulin can be considered as one of therapeutic options in this clinical condition.
This study has several limitations. First, only two studies with limited number of elderly patients were included in this study. Second, we did not assess the risk of adverse event, especially for gastrointestinal-related adverse event, which was more common in oral lefamulin group than moxifloxacin group. Further study is needed to investigate these issues. Third, subgroup analyses have well documented issues notable insufficient power and potential imbalances in prognostic factors. Therefore, we cannot conclude noninferior from our data based on the lack of a prespecified margin and the issues with subgroup analyses.
In conclusion, the rates of clinical response were similar between the lefamulin and moxifloxacin treatment groups in patients ≥65 years of age. For elderly patients with CABP, this novel agent—lefamulin could be a therapeutic alternative in this clinical entity.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-19-3805). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jackson ML, Neuzil KM, Thompson WW, et al. The burden of community-acquired pneumonia in seniors: results of a population-based study. Clin Infect Dis 2004;39:1642-50. [Crossref] [PubMed]
- Fung HB, Monteagudo-Chu MO. Community-acquired pneumonia in the elderly. Am J Geriatr Pharmacother 2010;8:47-62. [Crossref] [PubMed]
- Ewig S, Birkner N, Strauss R, et al. New perspectives on community-acquired pneumonia in 388 406 patients. Results from a nationwide mandatory performance measurement programme in healthcare quality. Thorax 2009;64:1062-9. [Crossref] [PubMed]
- Veve MP, Wagner JL. Lefamulin: Review of a Promising Novel Pleuromutilin Antibiotic. Pharmacotherapy 2018;38:935-46. [Crossref] [PubMed]
- Mendes RE, Farrell DJ, Flamm RK, et al. In Vitro Activity of Lefamulin Tested against Streptococcus pneumoniae with Defined Serotypes, Including Multidrug-Resistant Isolates Causing Lower Respiratory Tract Infections in the United States. Antimicrob Agents Chemother 2016;60:4407-11. [Crossref] [PubMed]
- Waites KB, Crabb DM, Duffy LB, et al. In Vitro Activities of Lefamulin and Other Antimicrobial Agents against Macrolide-Susceptible and Macrolide-Resistant Mycoplasma pneumoniae from the United States, Europe, and China. Antimicrob Agents Chemother 2017;61:e02008-16. [Crossref] [PubMed]
- File TM, Goldberg L, Das A, et al. Efficacy and Safety of Intravenous-to-oral Lefamulin, a Pleuromutilin Antibiotic, for the Treatment of Community-acquired Bacterial Pneumonia: The Phase III Lefamulin Evaluation Against Pneumonia (LEAP 1) Trial. Clin Infect Dis 2019;69:1856-67. [Crossref] [PubMed]
- Alexander E, Goldberg L, Das AF, et al. Oral Lefamulin vs Moxifloxacin for Early Clinical Response Among Adults With Community-Acquired Bacterial Pneumonia: The LEAP 2 Randomized Clinical Trial. JAMA 2019;322:1661-71. [Crossref] [PubMed]


