
Coronavirus disease 2019 pneumonia may present as an acute exacerbation of idiopathic pulmonary fibrosis
The coronavirus disease 2019 (COVID-19) pandemic has resulted in a global health emergency. Computed tomography (CT) features of COVID-19 are characterized by bilateral ground-glass opacity (GGO) and mixed consolidation with GGO in the subpleural lung regions (1). However, imaging findings of severe COVID-19 pneumonia overlap with those of accelerated deterioration in patients with interstitial lung disease (ILD) without severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (2). In addition, common symptoms of COVID-19 pneumonia include fever with respiratory symptoms, same as those of acute exacerbation (AE) of ILD (3). Therefore, it seems difficult to distinguish between idiopathic AE-ILD with no identified trigger and COVID-19 pneumonia developing in patients with ILD. Here, we report the case of a patient with COVID-19 pneumonia presented as an AE-ILD.
A 76-year-old man presented to the emergency room with a one-week history of cough and deteriorating dyspnea. He was diagnosed with idiopathic pulmonary fibrosis (IPF) approximately 5 years ago and was taking pirfenidone. Chest CT conducted 6 months ago revealed bilateral subpleural distribution of reticulation, traction bronchiectasis, and honeycombing (Figure 1A,B,C). He did not have a fever on admission, but developed a low-grade intermittent fever throughout the clinical course. The laboratory test results were as follows: white blood cell count, 6,690 cells/mm3 (lymphocytes, 9%); C-reactive protein (CRP), 10.53 mg/dL; procalcitonin, 0.082 ng/mL; and lactate dehydrogenase (LDH), 781 U/L. An initial chest radiograph showed diffuse air-space infiltrates in both whole lung fields. Chest CT showed diffuse GGO in both the lungs with sub-pleural predominance (Figure 1D,E,F). Based on his symptoms, underlying disease, and radiologic findings, AE-IPF was suspected initially. The patient was administered methylprednisolone 20 mg twice a day and empirical antibiotics including piperacillin/tazobactam and levofloxacin. To rule out infectious etiology, microbiological examination including serology tests, sputum culture, and a multiplex viral PCR test with nasopharyngeal swab were performed. At the same time, a real-time reverse transcriptase polymerase chain reaction (RT-PCR) test for SARS-CoV-2 was performed using a nasopharyngeal swab sample, which yielded a positive result. Lopinavir plus ritonavir and hydroxychloroquine were added to the treatment regimen, but the patient’s dyspnea and oxygen demand continued to get worse. Even though mechanical ventilation was required, he declined mechanical ventilation and gave a do-not-resuscitate order. He died on the 18th day of hospitalization while receiving supportive care.
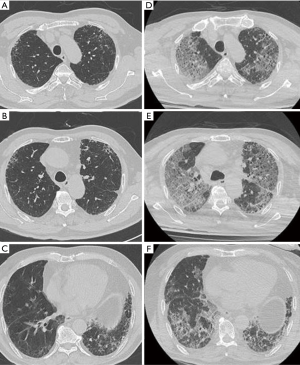
AE-ILD can occur at any time during the course of ILD and is associated with significant morbidity and mortality (4). Presenting symptoms are fairly similar between patients with AE-ILD and severe COVID-19 pneumonia. In addition, most patients with AE-ILD show increased inflammatory laboratory levels such as CRP and LDH (4), which are also increased in those with severe COVID-19 (3). The involvement patterns of newly appearing GGO with or without consolidation during AE-ILD are classified into peripheral, multifocal, and diffuse types (2,5). In particular, the peripheral pattern of parenchymal abnormalities makes differential diagnosis between the two diseases very challenging. Diffuse alveolar damage, a common pathologic finding that is observed in patients with AE-ILD and severe COVID-19, may be associated with these clinical and radiological findings (4,6).
According to recently proposed new diagnostic criteria of AE-IPF (7), our patient is likely to have a triggered AE-IPF due to SARS-CoV-2 infection. It is hard to determine whether the main pathogenesis of newly developed pulmonary lesions of this case is attributed to the triggered AE-IPF or COVID-19 pneumonia itself by clinical and radiological findings. Since the characteristics of COVID-19 in patients with ILD have not been well known, it was challenging to suspect SARS-CoV-2 infection immediately upon admission in this case. If treatment was continued only for the diagnosis of idiopathic AE-IPF without the RT-PCR testing to detect a SARS-CoV-2, nosocomial spread of COVID-19 may have been unavoidable. Further studies are needed to investigate whether a triggered AE-ILD by SARS-CoV-2 has clinical and radiologic characteristics that are distinguishable from those of COVID-19 pneumonia.
Nucleic acid test is a gold standard method for the confirmation of SARS-CoV-2 infection. However, some cases with false-negative results on real-time RT-PCR are reported (8). Thus, chest CT features play a complimentary role along with RT-PCR test in the diagnosis of COVID-19. However, the role of chest CT may be limited in these patients with ILD.
In conclusion, patients with underlying ILD may experience severe COVID-19, which has similar clinical and radiologic characteristics to AE-ILD. In areas where COVID-19 is prevalent, a thorough screening for the diagnosis of SARS-CoV-2 infection is important if a patient is clinically suspected with AE-ILD. Future studies on the clinical and radiological features are needed with a large subset of such patients.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1658). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 2020;20:425-34. [Crossref] [PubMed]
- Akira M, Hamada H, Sakatani M, et al. CT findings during phase of accelerated deterioration in patients with idiopathic pulmonary fibrosis. AJR Am J Roentgenol 1997;168:79-83. [Crossref] [PubMed]
- Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020;382:1708-20. [Crossref] [PubMed]
- Leuschner G, Behr J. Acute Exacerbation in Interstitial Lung Disease. Front Med (Lausanne) 2017;4:176. [Crossref] [PubMed]
- Akira M, Kozuka T, Yamamoto S, et al. Computed tomography findings in acute exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2008;178:372-8. [Crossref] [PubMed]
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420-2. [Crossref] [PubMed]
- Collard HR, Ryerson CJ, Corte TJ, et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am J Respir Crit Care Med 2016;194:265-75. [Crossref] [PubMed]
- Li D, Wang D, Dong J, et al. False-Negative Results of Real-Time Reverse-Transcriptase Polymerase Chain Reaction for Severe Acute Respiratory Syndrome Coronavirus 2: Role of Deep-Learning-Based CT Diagnosis and Insights from Two Cases. Korean J Radiol 2020;21:505-8. [Crossref] [PubMed]


