Adjuvant chemotherapy after resection of N1 non-small cell lung cancer: differential impact of new evidence on physician and patient decisions
Background
Lung cancer is the leading cause of cancer death and second most common primary site of malignancy for both genders, with non-small cell lung cancer (NSCLC) histology accounting for 85% of all cases (1). In the United States in 2012, an estimated 226,160 new cases of lung and bronchial cancer were diagnosed and 160,340 people died as a result of their disease. Most patients (56%) have metastatic disease at the time of diagnosis, while 15% of patients have disease confined to the primary site and 22% have disease involvement of regional lymph nodes. The 5-year relative survival is 52.2% for patients with localized disease at diagnosis and 25.1% for patients with regional lymph node spread (1). The standard for decades for patients with potentially resectable stages I-IIIA tumors has generally been referral for surgical consultation. Surgically resected stages IIA, IIB, and IIIA NSCLC confer 5-year survival rates of 46%, 36%, and 24%, respectively (2).
Published evidence over the past nine years has led to major changes in clinical guidelines regarding the use of adjuvant treatment in this situation. In 2004 and 2005, large randomized trials demonstrated a modest increase in 5-year survival of patients who received adjuvant cisplatin-based therapy (ACT) after complete surgical resection of stages I-IIIA NSCLC (3-5). A large meta-analysis in 2008 of these and other trials confirmed improved 5-year survival rates for stage II (10%) and IIIA (13%) (6). Currently, both the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) recommend ACT for patients with completely resected stage II or IIIA NSCLC (7-9).
Despite the demonstrated survival benefit of ACT, guideline adherence for adjuvant chemotherapy after NSCLC resection has been shown to be only 61.3-64% (9,10). The barriers to the use of ACT in a non-trial setting are not well understood, but are likely to include opinions of both physician and patient regarding ability to tolerate chemotherapy and whether the potential benefits of adjuvant therapy outweigh the risks. In order to better understand and quantify the practical use of ACT, we evaluated surgeon referral to medical oncology and oncologist recommendations for ACT following surgical resection of stages II-IIIA NSCLC with N1 nodal status from 2000-2012; N1 was singled out because chemotherapy use for N2 disease was already strongly supported by existing research. In this exploratory analysis, we sought to characterize the transition of clinical practice at our institution in light of new evidence and changing guidelines and to document reasons for non-referral to medical oncology or non-recommendation of ACT. We hypothesized that the new evidence resulted in increased adjuvant therapy recommendations.
Patients & methods
Data
Local institutional review board approval was obtained including waiver of consent. An internal, prospective database of all institutional thoracic surgery patients was queried for patients undergoing lobectomy or greater resection for N1 NSCLC during the study period, with additional chart review to complete data collection. Data elements extracted included demographics, neoadjuvant therapy status, significant comorbidities, intra-operative details, pathologic histology and stage, events of post-operative course, post-operative surgical notes, oncologic evaluation notes, and information on chemotherapy course, if applicable. Reasons for non-referral or ACT not recommended were categorically noted. Stage was recorded based on the American Joint Committee on Cancer, seventh edition, staging system; patients treated during times of earlier staging editions were recoded according to the 7th edition definitions (2).
Study cohort
The study cohort included patients older than 18 years of age who underwent resection of a single lobe or greater without neoadjuvant chemotherapy for pathologically confirmed N1 NSCLC between Jan 1st, 2000 and Aug 31st, 2012, and who attended at least one post-operative visit with an institutional surgeon.
Outcomes and covariates
Because administration of chemotherapy to patients during the study time frame would be only after direct evaluation by a medical oncologist, the outcomes of interest were referral by the surgeon to medical oncology, recommendation for ACT by the oncologist, and initiation of ACT within six months of surgical resection. Covariates of interest included demographics (age, sex, race), operative details (extent of resection and surgical approach), tumor characteristics (histopathology and staging), pre-operative pulmonary function, smoking history, post-operative performance status, and presence of major comorbidities (including coronary artery disease, congestive heart failure, diabetes mellitus, renal insufficiency, and history of stroke).
Statistical analysis
The analysis was stratified by date of surgery. Patients who underwent surgery between Jan 1st, 2000 and Dec 31st, 2005, were included in the 2000-2005 cohort, whereas patients who underwent surgery between Jan 1st, 2006 and Aug 31st, 2012 were included in the 2006-2012 cohort. The dates were chosen to account for dissemination into practice following the publications in 2004 and 2005 of the two major trials that demonstrated the benefit of ACT.
Patient characteristics, referral status, and recommendation for chemotherapy were tabulated for each cohort era. Performance status was categorized into good (Karnofsky 80-100 or ECOG 0-1), fair (Karnofsky 60-70 or ECOG 2), and poor (Karnofsky <60 or ECOG 3). Continuous data is presented as mean ± standard deviation unless otherwise indicated. Fisher’s exact test and chi-squared test of independence were used to compare referral and recommendation status between the cohort eras. Plotting referral to an oncologist by year visually demonstrated changes in referral practice over time, with referral frequencies compared using chi-squared tests of independence. In order to further evaluate whether era of surgery was an independent predictor of whether or not adjuvant chemotherapy was considered, a multivariable logistic regression model was created with “referral to medical oncology” as the outcome and “era of surgery” and patient age as potential predictors. These two predictors were chosen after considering the number of events and heterogeneity and number of missing variables for all potential predictors. A two-tailed probability value of less than 0.05 was considered significant. The data were imported into R Studio (Version 97.312) for analysis.
Results
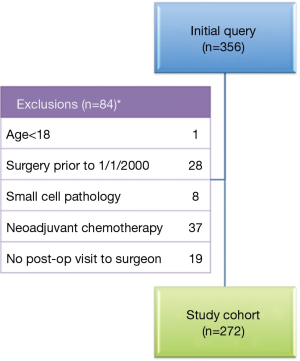
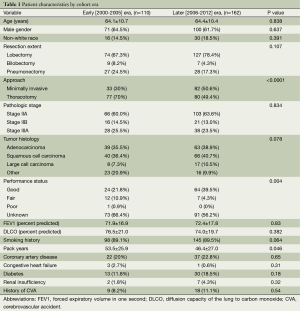
Initial query of the institutional database yielded 356 patients who underwent resection of lung cancer with N1 nodal disease. After exclusion of 84 patients (Figure 1), 272 patients who met inclusion criteria were identified. The 2000-2005 cohort included 110 patients and the 2006-2012 cohorts included 162 patients, as shown in Table 1. The patients in the two eras were similar in age, gender and racial distribution, pathologic stage, tumor histology, pulmonary function, and presence of comorbidities. The two cohorts shared similar proportions of patients with any tobacco use history, but patients of the early cohort had significantly greater mean pack-years compared to patients of the later cohort (53.5±25.9 vs. 46.4±27.0, P=0.046). Post-operative performance status was “good” in 39.5% of the later 2006-2012 cohort in contrast to only 21.8% of the early 2000-2005 cohort (P=0.004); however, data were only recorded for 56.2% and 66.4% of patients, respectively. Although the extent of resection (lobectomy vs. bilobectomy vs. pneumonectomy) was similar between the two cohorts, the proportion of patients undergoing minimally invasive procedures was significantly higher in the later cohort compared to the earlier cohort [50.6% (82 of 162) vs. 30% (33 of 110), P<0.001].
Full table
Frequency of patient referral to medical oncology by year is presented in Figure 2. Analysis of consecutive years demonstrated that the proportion of patients referred to medical oncology changed significantly from 72.4% in 2004 to 93.1% in 2005-2006 (χ2=4.35, P=0.037); this demonstrates a change in clinical practice one year before our categorical divider of 2006, selected a priori.
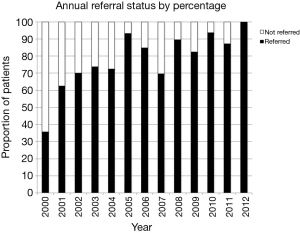
Figure 3 shows the referral and recommendation status of patients by cohort era. The proportion of patients referred to an oncologist increased from 74.5% in 2000-2005 to 90.1% in 2006-2012 (P=0.002). In addition in multivariable analysis, patients who had surgery in the later era were much more likely than patients in the early era to be referred to medical oncology to consider ACT [odds ratio 3.2, 95% confidence interval (CI): 1.6-6.2, P=0.0008]. Similarly, the proportions of evaluated patients recommended for ACT increased from 61% in the earlier cohort to 81.5% in the later cohort (χ2=16.1, P<0.001). For patients whose recommended chemotherapy regimens were documented, a platin-based doublet was the recommended chemotherapy regimen in the majority of patients in both eras [earlier era 97% (32 of 33 patients) vs. later era 96% (75 of 78 patients), P=1]. Of note, 25% (n=28) of patients in the early cohort era and 10% (n=16) of patients in the later cohort era were not referred for medical oncology evaluation after surgery. Among patients who were evaluated by a medical oncologist, the outcome of the oncologist’s evaluation was unknown for 13.4% (11 of 82) of patients in the early cohort era and 8.9% (13 of 162) of patients in the later cohort era.
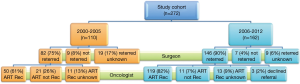
Prior to 2006, oncologists did not recommend ACT for 25.6% (21 of 82) of evaluated patients; lack of indication (12 of 21, 57%) was the most common reason given; the other reason was that the patient was not an adequate candidate (9 of 21, 43%). In the years 2006-2012, 7.5% (11 of 146) of evaluated patients were not recommended for ACT; most often the oncologist deemed the patient was not an adequate candidate (6 of 11, 54.5%), with ACT being deemed as not indicated in the remaining patients (5 of 11, 45.5%). Although oncologists recommended ACT to a higher percentage of patients in the later era, the percentages of patients who were offered but declined adjuvant treatment were similar between the two eras: 14% (7 of 50) of 2000-2005 patients vs. 13.4% (16 of 119) of 2006-2012 patients, (P=0.666), as shown in Table 2.

Full table
Table 3 lists the characteristics of patients who agreed to and who declined ACT. Overall, patients who declined chemotherapy were significantly older than patients who accepted therapy (68.4±9.4 vs. 62.6±9.2, P=0.010); none of the other baseline characteristics differed significantly between the groups. For patients 2006-2012, the age gap was even greater between those who declined (mean =70.0, SD =8.13) and those who accepted (70.0±8.1 vs. 61.9±9.4, P=0.002); other characteristics of the later cohort era and all characteristics of the earlier cohort era were not found to differ significantly between groups based on pursuit of ACT (data not shown). Of note, performance status was not different between these groups, though those data were available for less than half of each group.
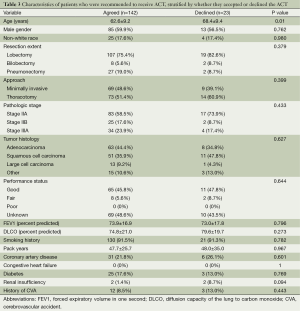
Full table
In the 2006-2012 cohort, 92.9% (92 of 103) patients who were recommended for and agreed to ACT were documented to initiate treatment, which was significantly higher than that observed in the earlier cohort (81.4%, 35 of 43 patients) (P=0.018), as shown in Table 4. In the later era, 60.1% (n=56) of the 92 patients documented to receive ACT were treated by the oncologist who initially recommended therapy and 39.9% (n=36) were treated by an oncologist who practiced closer to where the patient lived. In the early era, roughly equal proportions of patients were treated by the oncologist who made the initial recommendation [54.3% (19 of 35)] and by an oncologist who practiced closer to where the patient lived [45.7% (16 of 35)].

Full table
Discussion
This study characterizes the management of patients who had undergone resection of NSCLC with N1 nodal disease at one institution from 2000 to 2012, a period during which several phase III clinical trials demonstrated significant survival benefit of ACT for this patient population. The analysis shows that clinical practice by both surgeons and oncologists significantly changed in response to newly published evidence, even ahead of the predetermined year divider. The somewhat earlier than expected change in clinical practice may indicate that evidence dissemination from abstract presentations and research conferences can lead to rapid change in clinical practice even in advance of formal publication. In this study, increasing referral rates from 74.5% in 2000-2005 to 90.1% in 2006-2012 were accompanied by an increase the percentage of patients who were recommended by medical oncology to be given ACT (60.1% in 2000-2005 vs. 81.5% in 2006-2012). These findings suggest a successful dissemination of practice-changing research prior to updates of official guidelines. In addition, a greater proportion of patients who agreed to ACT were documented to have actually initiated treatment in the later cohort (92.9%) compared to the earlier cohort (81.4%), proportions which are higher than recent research suggests (10,11). Had the categorical dividing year been 2005 (as the data shows) instead of 2006 (selected a priori), the magnitude of difference between cohort eras of referral practices, oncologists’ recommendations, and initiation of ACT might have been even more extreme. This result may indicate that physicians at an academic medical center are likely to incorporate new research data into practice more quickly than in other practice situations. However, chemotherapy referrals were never 100% annually, as some patients were lost to follow up and others were considered too weak to pursue chemotherapy post-operatively.
In our study, the most common documented reason for ACT not being delivered was poor performance status. The use of induction chemotherapy followed by surgical resection may be a better strategy in some patients whose ability to tolerate surgery could improve with pulmonary rehabilitation or with better control of concomitant medical conditions. In addition, patient selection and surgical treatment must be optimized such that patients have the best chance of avoiding morbidity and maintaining an adequate performance status for a course of ACT. In this regard, the use of minimally invasive surgical techniques has been demonstrated to improve the compliance of ACT (12,13). Improved ACT compliance would allow for more clear evaluation of long term patient outcomes, whereas patients’ cessation of treatment or dose reduction due to patient choice or toxicity hinder such evaluation in a non-trial setting (14).
One important implication of our study is that new evidence clearly has different impacts on physicians and patients. The proportion of patients who were offered but declined ACT did not change significantly between the two eras, despite the increased rate of referral to medical oncology post-surgery and the increased rate of medical oncology recommending chemotherapy. Interestingly, the proportion of patients who refused treatment in our study (13-14%) was smaller than in an earlier, multi-centered study, where 26% of patients refused further treatment (15). Although we are not able to delineate precise reasons for why patients declined ACT, it is likely that some patients did not feel the risks of ACT were worth the potentially increased chance of long-term survival. It is important to note that new evidence did not impact patient decision-making in nearly the same degree that physician decision-making was impacted. Better understanding of the patient’s decision-making process is clearly needed, given that research has suggested that physicians often misunderstand patient attitudes towards chemotherapy. Moreover, in the setting of emotional distress due to a new cancer diagnosis, patients are asked to weigh numerically defined risks versus benefits and process complex information (16). The aforementioned tasks confound the average American who reads at the eighth grade level and even 20% of college-educated adults cannot identify the higher risk percentage: 1%, 5%, or 10% (17). Given these challenges to effective communication, the medical literature and wider community would benefit from careful documentation of the patient-physician decision-making process, including analysis of patients’ comprehension of options, risks, and benefits, and individual goals of therapy and preferences.
Another important implication of our study is that any benchmarks that are set in regards to ACT use for NSCLC must recognize that some small but significant percentage of patients will choose to decline treatment recommendations. In the context of evaluating system performance measures, care coordination, and effectiveness, the proportion of patients not being definitively evaluated (35.5% in 2000-2005 and 17.9% in 2006-2012) or declining treatment (14.0% in 2000-2005 and 13.4% in 2006-2012) highlights the fact that organizations may not be able to implement guideline-driven treatment for all patients (18). Additionally, one-third to one-half of patients undergoing evaluation at our tertiary medical center were treated by an oncologist outside of this health system who was closer to the patient’s home. These findings are important in an environment in which reimbursement may become more closely tied to adequate documentation of quality of care. Clearly, the care that patients ultimately agree to receive goes beyond just what the evidence suggests is associated with the best outcomes.
There are several important limitations to be recognized. First, the data were from a single site and may not generalize to other institutions. In addition, the findings of our study cohort of United States patients may not be as applicable to cohorts of patients in other countries, where the administration of chemotherapy may be directly by surgeons without medical oncology referral and where the distribution of patient characteristics such as smoking and lung cancer histology may be different than those observed in our cohort. Second, this study was limited by both the retrospective nature of the study and the small sample size, especially with respect to comparisons of patients who did or did not receive ACT. In addition, explicit documentation of chemotherapy evaluation, recommendations, and administration were not consistently available for review for all patients. Therefore, our findings could be biased if there were any unmeasured characteristics of the patients whose follow-up was not complete which were different from the patients for whom we had complete follow-up. In particular, performance status data were absent for over half of our patients. Considering that performance status is generally a very critical determinant when deciding if chemotherapy is appropriate for a patient, our study findings could potentially be biased if the distribution of performance statuses of the patients for whom the data was missing had more patients with worse performance status than what we observed in the patients for whom performance status had been recorded. Finally, one-third to one-half of patients were followed or treated by their local oncologist. As a result, clinical details were also not available for patients who were not followed after surgery or who choose to undergo ACT at an outside institution; missing data were treated as “unknown” in our analysis.
In conclusion, referrals and recommendations for ACT use increased following the publication of supporting evidence but did not significantly change the percentage of patients who ultimately agreed to receive ACT. Our research supports that new evidence leads to significant change in practice but it also highlights also the need to understand how patients make decisions about their cancer care. Future studies that have a greater number of patients or prospectively evaluated ACT will demonstrate if the changes in referrals and recommendations were not unique to our institution and hopefully clarify patient decision making as well as further define realistic goals for the use of ACT.
Acknowledgements
We wish to thank the Duke Tumor Registry for their support in this study.
Authors’ contributions: Brooke K. Coleman co-designed the overall study, collected and analyzed data, and wrote the manuscript. Lesley H. Curtis co-supervised the study and contributed to the analysis and interpretation of data, and critically revised the manuscript. Mark W. Onaitis and Thomas A. D’Amico contributed to the interpretation of data and critically revised the manuscript. Mark F. Berry co-designed the study, co-supervised the study, collected, analyzed and interpreted data, and critically revised the manuscript. All authors approved the final version submitted.
Funding: This work was supported by the National Institute of Health-funded Cardiothoracic Surgical Trials Network [5U01HL088953-05 to M.F.B].
Disclosure: The authors declare no conflict of interest.
References
- Howlader N, Noone AM, Krapcho M, et al. eds. SEER Cancer Statistics Review, 1975-2009 (Vintage 2009 Populations). Bethesda, MD: National Cancer Institute, 2012.
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [PubMed]
- Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004;350:351-60. [PubMed]
- Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 2006;7:719-27. [PubMed]
- Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 2005;352:2589-97. [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [PubMed]
- Ettinger DS, Akerley W, Borghaei H, et al. Non-small cell lung cancer. J Natl Compr Canc Netw 2012;10:1236-71. [PubMed]
- Pisters KM, Evans WK, Azzoli CG, et al. Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I-IIIA resectable non small-cell lung cancer guideline. J Clin Oncol 2007;25:5506-18. [PubMed]
- Wang Z, Askamit I, Tuscher L, et al. Rates of guideline adherence among US community oncologists treating NSCLC. Am J Manag Care 2013;19:185-92. [PubMed]
- Booth CM, Shepherd FA, Peng Y, et al. Adoption of adjuvant chemotherapy for non-small-cell lung cancer: a population-based outcomes study. J Clin Oncol 2010;28:3472-8. [PubMed]
- Kankesan J, Shepherd FA, Peng Y, et al. Factors associated with referral to medical oncology and subsequent use of adjuvant chemotherapy for non-small-cell lung cancer: a population-based study. Curr Oncol 2013;20:30-7. [PubMed]
- Lee JG, Cho BC, Bae MK, et al. Thoracoscopic lobectomy is associated with superior compliance with adjuvant chemotherapy in lung cancer. Ann Thorac Surg 2011;91:344-8. [PubMed]
- Petersen RP, Pham D, Burfeind WR, et al. Thoracoscopic lobectomy facilitates the delivery of chemotherapy after resection for lung cancer. Ann Thorac Surg 2007;83:1245-9;discussion 1250. [PubMed]
- Carbone DP, Felip E. Adjuvant therapy in non-small cell lung cancer: future treatment prospects and paradigms. Clin Lung Cancer 2011;12:261-71. [PubMed]
- Zornosa C, Mamet R, Reid ME, et al. Utilization of adjuvant therapy among completely resected non-small cell lung cancer (NSCLC) patients in the National Comprehensive Cancer Network (NCCN) Outcomes Database Project. J Clin Oncol 2010;abstr 7017.
- Fagerlin A, Zikmund-Fisher BJ, Ubel PA. Helping patients decide: ten steps to better risk communication. J Natl Cancer Inst 2011;103:1436-43. [PubMed]
- Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Making 2001;21:37-44. [PubMed]
- McClellan M, McKethan AN, Lewis JL, et al. A national strategy to put accountable care into practice. Health Aff (Millwood) 2010;29:982-90. [PubMed]


