Accelerated radiotherapy and concurrent chemotherapy for patients with contralateral central or mediastinal lung cancer relapse after pneumonectomy
Introduction
Patients with lung cancer who experience isolated contralateral central intrapulmonary or mediastinal relapse following pneumonectomy may be considered for definitive salvage therapy. Results of small surgical series for very selected patients have been reported but since resections were performed with narrow margins, patients mainly died of pulmonary recurrences after surgical re-treatment (1,2). The majority of patients selected for surgery presented with limited tumour burden after primary treatment. The therapeutic window for salvage-resection is highly restricted and radiotherapy offers the only alternative local treatment option with curative potential. In patients with stage III tumours, initial treatment often requires pneumonectomy when surgery is used within multimodality treatment concepts including neoadjuvant radiochemotherapy. Pneumonectomies were performed in about 20-35% of patients in larger treatment series on neoadjuvant radiochemotherapy for stage III patients (3-5). About 30% of patients treated with neoadjuvant radiochemotherapy at our institution have received pneumonectomy (6). This cohort included patients with selected IIIB tumours who have been offered surgery within trimodality trials (4,6-8).
Isolated intra-thoracic recurrences after trimodality were seen in about 10% of patients treated with pneumonectomy either after neoadjuvant radiochemotherapy in several series or with pneumonectomy alone as initial loco-regional treatment (5,7-10).
No sufficient data exist, whether retreatment of central contralateral intrapulmonary or mediastinal recurrences with definitive radiochemotherapy can offer a second curative chance with prolonged survival. Prior radiotherapy given adjuvant or neoadjuvant may further complicate the treatment situation. Improved radiotherapy techniques increase the options of reirradiation (11). With increasing evidence of stereotactic reirradiation or other options of chemoradiation as salvage treatment, the issue of rising toxicity becomes obvious (12,13).
Here, we present results from a group of patients with contralateral centrally located or mediastinal tumour recurrences who were treated after pneumonectomy by an intensive salvage chemoradiotherapy regime including accelerated radiotherapy [accelerated fractionated (AF), 8×2 Gy per week]. This schedule was intended to minimize normal tissue complications by using conventional doses per fraction while simultaneously increasing the biological effective dose using accelerated fractionation.
Methods
This retrospective analysis included consecutive patients treated since 10/2011 with histopathologically proven contralateral mediastinal or central intrapulmonal relapse after initial pneumonectomy for non-small cell lung cancer (NSCLC) with definitive accelerated radiochemotherapy. Four additional patients with peripheral intrapulmonary tumour relapses who have been treated by stereotactic radiotherapy as well as two further patients requiring central airway stents have been excluded from this analysis.
All patients were staged by 18F-Fluoro-Deoxyglucose-whole-body positron emission tomography and brain MRI before treatment start.
Concurrent radiochemotherapy was planned for weekly cisplatin (30 mg/m2) and 60 Gy accelerated radiotherapy. In order to avoid excess toxicity at relapse treatment, especially with respect to the esophageal mucosa and not to compromise the AF radiotherapy single-agent cisplatin as radiosensitizer was chosen based on earlier evidence (14,15). Induction therapy consisting of two to three cycles of cisplatin-based chemotherapy was allowed but not mandatory. Written informed consent was obtained from all patients.
Radiotherapy was planned in either free-breathing (tomotherapy) or deep inspiration breath-hold [multi-field intensity-modulated radiotherapy (IMRT)]. The internal target volume (ITV) was constructed from a 4-dimensional planning CT (SOMATOM Sensation® Open, Siemens, Erlangen, Germany) for irradiation in free breathing or three separate CT scans acquired during three separate inspiration breath-hold maneuvers over a time period of 10 min. An additional margin of 5-10 mm was added to create the planning target volume (PTV).
A total dose of 60 Gy was delivered in 8×2 Gy per week (Mon/Wed/Fri: 2×2 Gy/d, Tue/Thu: 1×2 Gy/d) representing a biologically effective dose (BED) >70 Gy.
Patients were treated using multi-field IMRT or helical tomotherapy, respectively. The decision for the appropriate technique was based on comparative plan ranking with respect to improved sparing of organs at risk by achievement of steeper gradients or advantages of using deep-inspiration breath-hold in upper lobe and central tumours, respectively.
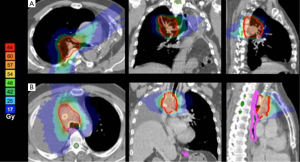
Beside target volume coverage, plans were optimized to minimize lung exposure, dose to spinal cord and esophageal mucosa with steepest dose gradients towards the nearest dose-limiting organ at risk. Constraints to organs at risk that were considered to be tolerable at a risk of severe late effects <5% were: lung: V20<10%, mean dose to the remaining lung <8 Gy; life-time spinal cord maximum dose: below 55 Gy, esophagus: cumulative Dmax (contralateral wall, including doses of former radiotherapy) <80 Gy; trachea and major vessels (contralateral wall, former radiotherapy doses included) <80 Gy (16-18).
Image guidance during treatment was performed with on-board imaging devices and on-line cine verifications; pretreatment set-up was controlled with orthogonal portal images or cone-beam-CTs.
The 3D-dose distribution and dose volume histograms were optimized by the treatment planning system of the manufacturers of the treatment machines (ECLIPSE®, Varian Medical Systems, Tomo Hi-ART® Planning Station), 6-15 MV photons were used.
After treatment, patients were seen at regular 3-month intervals including clinical examination, chest CT, or chest X-ray. Radiographic changes were classified according to the scoring system suggested by Palma et al. (19).
Data analysis
Statistical analyses were performed using the SAS software package (SAS® Institute Inc., Cary, NC, USA). Survival curves (time to progression, overall survival) have been calculated according to the method of Kaplan-Meier.
Results
Between 10/2011 and 12/2013, seven patients, six males, one female received accelerated radiotherapy as depicted above. Median age was 61 years, range 48 to 73 years. The performance status of these patients was WHO I in three and WHO II in four patients, respectively. Initial stages (before pneumonectomy) were IIB in three patients, IIIA in one patient, IIIB in three patients. Of these patients, three had received neoadjuvant chemoradiotherapy including cisplatin/paclitaxel combination chemotherapy. One patient received postoperative treatment including carboplatin/ vinorelbine and radiotherapy. Two patients did not receive planned adjuvant treatment due to comorbidities and patient refusal.
All tumour recurrences were proven by either cytology or histology. Tumours showed moderate (n=4) or poor (n=3) differentiation. Squamous cell histology was diagnosed in four, adenocarcinoma in one, large cell carcinoma in two patients, respectively. No mutations of the epidermal growth factor (EGF)-receptor gene were found in the adenocarcinoma. Mean tumour SUVmax in the 18F-FDG-PET/CT scans was 18.1 (range, 9-28.6).
Median interval between pneumonectomy and relapse was 18 [12-96] months. Tumour relapses were classified as rT0 N0 M1a in two patients, rT0 N3 M1a in two patients, rT0 N3 M0 in two patients, rT3 N2 M0 in one patient. In terms of bronchoscopic findings, in four patients tumours were within 2 cm close to the carina while in one patient the contralateral intrapulmonary tumour was located within the zone of the contralateral proximal bronchial tree at the intermediate bronchus more than 2 cm away from the tracheal carina. Pre-reirradiation FEV1 was 1.38 L/s (45%) on average (range, 1.17-1.5 L/s, 37-52%), mean diffusion capacity was 51.2% (32-65%).
Four patients have received induction chemotherapy (cisplatin/paclitaxel, n=3, cisplatin/pemetrexed, n=1) after diagnosis of relapse. During salvage-radiotherapy, two patients refused concurrent chemotherapy, all others received the intended cycles of weekly cisplatin.
Radiotherapy
Parameters of radiotherapy dose-volume exposure for lung, spinal cord, and esophagus are presented in detail in Table 1. Average V20 was 13.2% (range, 3.8-18.4%), mean total lung dose was 7.11 Gy on average (range, 3.3-11.4 Gy). Median conformity index was 1.18 (range, 1.04-1.55), homogeneity indices ranged from 0.08-0.25 (median 0.18).
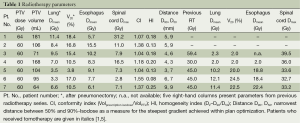
Full table
Five patients have had previous radiotherapy. Total doses were 30 Gy in one, 45 Gy in three, and 59.4 Gy in one patient. The steepest achieved dose gradients perpendiculars to the surface of the PTV are given in Table 1 (see also Figure 1). In five patients, the esophagus abutted directly to the CTV. The achieved maximum doses at the contralateral esophageal wall were 10.0-58.5% (median 48.3%) of the prescribed dose.
Toxicity
Hematologic toxicity of concurrent radiochemotherapy was mild and did not exceed grade 2 Common Toxicity Criteria (CTC). With a median follow-up of 33 [21-36] months, no clinically relevant esophageal toxicity (> grade 2 CTC) was observed.
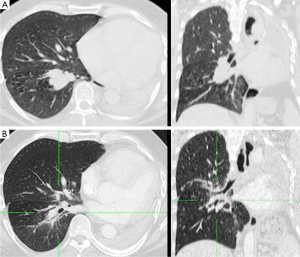
Clinical findings of pneumonitis grade 2 CTC were observed in two patients, grade 1 in three, and grade 0 in two patients, respectively. Post-radiotherapy FEV1 remained at 40% on average (range, 35.6-46%). One patient needed an interventional bronchoscopy three days after the end of radiotherapy due to viscous mucus leading to obstructive respiratory insufficiency. Complete recovery was achieved thereafter. In their last imaging study at a median interval of 10 months (range, 4-24 months) after treatment two patients showed no radiation induced lung abnormalities on radiographic examinations, one patient showed diffuse ground-glass opacities in a margin of 2.5 cm around the PTV, three patients had patchy consolidations smaller than 2 cm within a margin of 1.5 cm around the PTV. Only one patient presented with diffuse consolidations within a margin of 2.5 cm around the PTV at 10 months after treatment.
Patterns of failure and survival data
At three months, all tumours showed partial response (Figure 2). Actuarial median progression-free survival was 10.4 months. First sites of relapse were distant failures [brain (n=1) and liver (n=2)], which in two cases were associated with regional out-field mediastinal lymph node recurrences, and regional out-field supraclavicular lymph node recurrence in one patient. Local in-field control at 12 months was 80%. Two local in-field recurrences were observed at 8 and 16 months after treatment, one in combination with regional mediastinal out-field failure, the other with regional mediastinal out-field and distant hepatic progression. Both patients have had stage IIIB tumours at initial diagnosis.
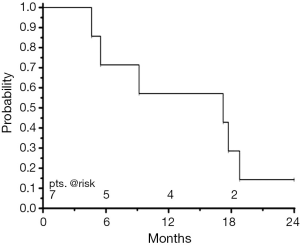
Median overall survival of all patients was 17.2 (95% CI: 4.6-18.8) months, the overall survival rate at one year was 57% (Figure 3). Two patients have died following out-field tumour progression, one patient died 5 months after start of treatment due to a community-acquired lobar bacterial pneumonia without detection of local recurrence. One patient died 17 months after treatment following cerebral ischemia without proven evidence of local or distant disease. Currently (Sep 15, 2014), one patient is still alive. Of three patients surviving more than 12 months, one had received induction chemotherapy at the time of relapse. Two of these longer-term survivors got reirradiation at the time of relapse as they had radiotherapy as a part of their first-line treatment for the initial tumour.
Discussion
Here we report the results of an intensified chemoradiotherapy regime using AF radiotherapy for patients with contralateral intrapulmonary or mediastinal relapses after pneumonectomy for locally advanced NSCLC. This schedule was designed to minimize repopulation during therapy and to spare late effects by using conventional doses per fraction for a gain from different fractionation sensitivities of tumours and late reacting normal tissues. Applying eight times 2 Gy per week allows to reach 60 Gy in 4 weeks which represents a BED at the tumour of 70 Gy with conventional fractionation using an alpha/beta ratio of 10 Gy, repopulation rate 0.60 Gy/day and lag time tk=21 days (20). Accelerated fractionation and concurrent chemotherapy has been introduced as first line treatment (21). A similar schema using accelerated radiotherapy alone after induction chemotherapy to 44 Gy at 2×2 Gy per day has been used in the neoadjuvant setting and yielded 39% pathological remissions in the mediastinum (8). Our group has reported a rate of 37% pathologic complete remissions after accelerated hyperfractionation in the neoadjuvant setting higher than with conventional fractionation to the same total dose (6).
Tolerances of lung paremchyma are reduced after pneumonectomy. Trials on postoperative hemithorax irradiation after pleuropneumonectomy for malignant pleural mesothelioma found that the mean lung dose to the remaining lung should be <8 Gy in order to avoid severe pulmonary toxicity (17). This increased sensitivity makes highly conformal radiotherapy techniques an essential prerequisite for retreatment after pneumonectomy. In addition, reirradiation of centrally located tumours has to spare central mediastinal structures adjacent to the target volume, i.e., spinal cord, esophagus, major vessels, and proximal bronchial tree (22). The maximum dose gradients towards critical normal tissues achieved in this treatment series are comparable to those in stereotactic body radiotherapy (SBRT) for spherical volumes of similar size (23). However, to achieve such steep dose gradients in SBRT homogeneity within the target volume is reduced and dose maxima of >120% are allowed. Apart from two patients, all tumours in our group presented with mediastinal involvement close to the aforementioned organs at risk. Furthermore, 50% of our patients had PTV volumes >100 cc. Hypofractionation together with accepted dose inhomogeneities of SBRT would lead to an unacceptable risk of side effects in the mediastinum. There have been some reports pointing to this fact. Cannon et al. reported grade 4 to 5 toxicities in 6 of 79 patients treated within a dose-escalated hypofractionated radiotherapy (57 to 85.5 Gy, 25 fractions) phase I trial (24). The observed toxicities were mainly attributable to damage to central and perihilar structures correlating with dose to the proximal bronchial tree. For combined modality treatment, Roach and colleagues have underscored that patients receiving daily fractions greater than 2.67 Gy are at higher risk of radiation pneumonitis. This effect can be reduced by twice-daily treatment with lower doses per fraction (25). The fact that only one out of seven patients in our cohort developed diffuse consolidations after treatment with our accelerated 2 Gy per fraction regime is in contrast to recent analyses of stereotactic radiotherapy where diffuse consolidations have been found in up to 32% of patients after stereotactic radiotherapy and supports the assumption of a high fractionation sensitivity of the lung (19).
Monoinstitutional experience has been reported on stereotactic radiotherapy for new early-stage lung cancer arising post pneumonectomy (26-28). Senthi et al. have reported seven patients treated with hypofractionated or conventionally fractinonated radiotherapy for centrally located relapses post pneumonectomy (28). Median PTV volume was 27 cc. Three of four patients receiving hyopfractionated radiotherapy with 12×5 Gy developed grade 3+ pneumonitis. On the contrary, none of the four patients receiving 13 or more fractions up to 60 Gy in 30 fractions developed clinically relevant pneumonitis despite central location of their tumour and larger PTV volumes. This experience points to the normal tissue sparing effect of conventional doses per fraction in comparison to hypofractionation.
Peulen and colleagues reported a series of patients with stereotactic reirradiation after prior lung SBRT for 11 centrally located lung tumours up to 2 cm towards the periphery from the respective lobar carina according to the Radiation Therapy Oncology Group schema of the proximal bronchial tree (22,29). Those patients did not have major surgery. Grade 4 and 5 toxicity was observed in 2 and 3 of these patients, while none of peripheral retreated tumours experienced grade 4+ toxicity. These data underscore that retreatment tolerance to very high BEDs of the central bronchial zone is confronted with limits.
Most of our patients presented with stage IIIB or IVa tumours at relapse even when initially (before pneumonectomy) staged as IIB tumours. While 5-year survival rates after complete resection are reported in the range of 24-36% for stage IIB or IIIA tumours disease patients with locally advanced tumours at initial diagnosis face a prognosis of 36% survival at 2 years and 15% at 5 years after concurrent chemoradiation which is very similar to resected patients who unexpectedly turn out to have stage III disease after pneumonectomy (30-32). Recurrence leads to substantial survival reduction with median postrecurrent survival times between 8-18 months (30). Patients presenting at relapse with tumours at the border between advanced stage IIIB and oligometastatic disease, face higher concurrent risks of distant progression which is taken as a rationale for systemic therapy [e.g., platinum doublet chemotherapy after EGFR and anaplastic lymphoma kinase (ALK) testing] rather than local treatment. There is growing evidence, however, that additional aggressive local treatment yields favorable survival rates with median survival >13 months and 2-year survival rates >30% (33).
Kruser and colleagues have reported results of reirradiation in 37 NSCLC patients with the majority presenting with stage III tumours at initial diagnosis (34). Median survival was only 5.1 months after retreatment. The group from MD Anderson Cancer Center used protons for retreatment of NSCLC patients with intrathoracic recurrence (35). Twenty-eight of 33 patients received retreatment for a centrally located tumour, median ITV was 95.8 cc. Median overall survival of 31 patients who completed treatment was 11.1 months and severe pulmonary toxicity (≤ grade 3) was observed in 21% of the patients. The median survival of 17 months seen in the presented cohort of our institution is satisfactory and approaches the level of median survival from first line treatment in recent meta-analyses for simultaneous radiochemotherapy in locally advanced NSCLC (32,36,37).
The presented series of our institution is small and comprises a range of different tumour stages. The chance of cure remains limited in the more advanced tumour stages according to the current evidence suggesting that those patients with combined contralateral pulmonary and mediastinal lymph node involvement at recurrence have a median survival of less than 6 months and a 2-year survival rate of about 10% (38). Our patients had a median disease-free interval after pneumonectomy of 18 months. The longest initial disease-free periods (>45 months) were observed in the stage IIB patients. This represents a patient cohort where 2- and 5-year survival rates above 60% following additional local treatment after relapse have been achieved (38). The present and other studies, including a meta-analysis, suggest that in some patients with oligometastatic NSCLC, long-term survival may be achievable most often in the context of metachronous oligometastasis and a low intrathoracic disease burden (39,40). After early closure of two randomized trials, one additional trial remains ongoing (NCT01725165), comparing local consolidative therapy with no local therapy after chemotherapy for oligometastatic NSCLC. While awaiting such data, treatment decision making should involve a multidisciplinary thoracic oncology team and a well-informed patient, balancing the benefits with risks of local ablative therapy for oligometastatic NSCLC.
Conclusions
This intensified accelerated chemoradiotherapy schedule was safely applicable and offers a curative chance in these pretreated frail lung cancer patients.
Based on our initial experiences, we plan a phase II trial on the presented schedule for pretreated patients with limited alternative local salvage treatment options. This includes patients with contralateral central or mediastinal relapse after pneumonectomy, or neoadjuvant chemoradiotherapy and lung sparing resection, or definitive chemoradiotherapy for stage III lung cancer, respectively, after interdisciplinary counselling for salvage surgery options.
Acknowledgements
Authors’ contributions: C Pöttgen had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. C Pöttgen, M Stuschke, J Abu Jawad, E Gkika, T Gauler, and WE Eberhardt have made substantial contributions to conception and design, acquisition of data, and analysis and interpretation of data. L Freitag, W Lübcke, S Welter, M Stuschke, M Schuler, G Stamatis have drafted the submitted article and revised it critically for important intellectual content; all authors have approved the final version of the manuscript.
Disclosure: The authors declare no conflict of interest.
References
- Spaggiari L, Grunenwald D, Girard P, et al. Cancer resection on the residual lung after pneumonectomy for bronchogenic carcinoma. Ann Thorac Surg 1996;62:1598-602. [PubMed]
- Terzi A, Lonardoni A, Scanagatta P, et al. Lung resection for bronchogenic carcinoma after pneumonectomy: a safe and worthwhile procedure. Eur J Cardiothorac Surg 2004;25:456-9. [PubMed]
- Weder W, Collaud S, Eberhardt WE, et al. Pneumonectomy is a valuable treatment option after neoadjuvant therapy for stage III non-small-cell lung cancer. J Thorac Cardiovasc Surg 2010;139:1424-30. [PubMed]
- Stamatis G, Djuric D, Eberhardt W, et al. Postoperative morbidity and mortality after induction chemoradiotherapy for locally advanced lung cancer: an analysis of 350 operated patients. Eur J Cardiothorac Surg 2002;22:292-7. [PubMed]
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379-86. [PubMed]
- Pöttgen C, Eberhardt W, Graupner B, et al. Accelerated hyperfractionated radiotherapy within trimodality therapy concepts for stage IIIA/B non-small cell lung cancer: Markedly higher rate of pathologic complete remissions than with conventional fractionation. Eur J Cancer 2013;49:2107-15. [PubMed]
- Eberhardt W, Wilke H, Stamatis G, et al. Preoperative chemotherapy followed by concurrent chemoradiation therapy based on hyperfractionated accelerated radiotherapy and definitive surgery in locally advanced non-small-cell lung cancer: mature results of a phase II trial. J Clin Oncol 1998;16:622-34. [PubMed]
- Stupp R, Mayer M, Kann R, et al. Neoadjuvant chemotherapy and radiotherapy followed by surgery in selected patients with stage IIIB non-small-cell lung cancer: a multicentre phase II trial. Lancet Oncol 2009;10:785-93. [PubMed]
- Takeda S, Maeda H, Koma M, et al. Comparison of surgical results after pneumonectomy and sleeve lobectomy for non-small cell lung cancer: trends over time and 20-year institutional experience. Eur J Cardiothorac Surg 2006;29:276-80. [PubMed]
- Lopez Guerra JL, Gomez DR, Lin SH, et al. Risk factors for local and regional recurrence in patients with resected N0-N1 non-small-cell lung cancer, with implications for patient selection for adjuvant radiation therapy. Ann Oncol 2013;24:67-74. [PubMed]
- Griffioen GH, Dahele M, de Haan PF, et al. High-dose, conventionally fractionated thoracic reirradiation for lung tumors. Lung Cancer 2014;83:356-62. [PubMed]
- Jeremić B, Videtic GM. Chest reirradiation with external beam radiotherapy for locally recurrent non-small-cell lung cancer: a review. Int J Radiat Oncol Biol Phys 2011;80:969-77. [PubMed]
- McAvoy S, Ciura K, Wei C, et al. Definitive reirradiation for locoregionally recurrent non-small cell lung cancer with proton beam therapy or intensity modulated radiation therapy: predictors of high-grade toxicity and survival outcomes. Int J Radiat Oncol Biol Phys 2014;90:819-27. [PubMed]
- Schaake-Koning C, van den Bogaert W, Dalesio O, et al. Effects of concomitant cisplatin and radiotherapy on inoperable non-small-cell lung cancer. N Engl J Med 1992;326:524-30. [PubMed]
- Soresi E, Clerici M, Grilli R, et al. A randomized clinical trial comparing radiation therapy v radiation therapy plus cis-dichlorodiammine platinum (II) in the treatment of locally advanced non-small cell lung cancer. Semin Oncol 1988;15:20-5. [PubMed]
- Marks LB, Bentzen SM, Deasy JO, et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys 2010;76:S70-6. [PubMed]
- Miles EF, Larrier NA, Kelsey CR, et al. Intensity-modulated radiotherapy for resected mesothelioma: the Duke experience. Int J Radiat Oncol Biol Phys 2008;71:1143-50. [PubMed]
- Kirkpatrick JP, van der Kogel AJ, Schultheiss TE. Radiation dose-volume effects in the spinal cord. Int J Radiat Oncol Biol Phys 2010;76:S42-9. [PubMed]
- Palma DA, Senan S, Haasbeek CJ, et al. Radiological and clinical pneumonitis after stereotactic lung radiotherapy: a matched analysis of three-dimensional conformal and volumetric-modulated arc therapy techniques. Int J Radiat Oncol Biol Phys 2011;80:506-13. [PubMed]
- van Baardwijk A, Bosmans G, Bentzen SM, et al. Radiation dose prescription for non-small-cell lung cancer according to normal tissue dose constraints: an in silico clinical trial. Int J Radiat Oncol Biol Phys 2008;71:1103-10. [PubMed]
- Ball D, Bishop J, Smith J, et al. A randomised phase III study of accelerated or standard fraction radiotherapy with or without concurrent carboplatin in inoperable non-small cell lung cancer: final report of an Australian multi-centre trial. Radiother Oncol 1999;52:129-36. [PubMed]
- Peulen H, Karlsson K, Lindberg K, et al. Toxicity after reirradiation of pulmonary tumours with stereotactic body radiotherapy. Radiother Oncol 2011;101:260-6. [PubMed]
- Levegrün S, Pöttgen C, Wittig A, et al. Helical tomotherapy for whole-brain irradiation with integrated boost to multiple brain metastases: evaluation of dose distribution characteristics and comparison with alternative techniques. Int J Radiat Oncol Biol Phys 2013;86:734-42. [PubMed]
- Cannon DM, Mehta MP, Adkison JB, et al. Dose-limiting toxicity after hypofractionated dose-escalated radiotherapy in non-small-cell lung cancer. J Clin Oncol 2013;31:4343-8. [PubMed]
- Roach M 3rd, Gandara DR, Yuo HS, et al. Radiation pneumonitis following combined modality therapy for lung cancer: analysis of prognostic factors. J Clin Oncol 1995;13:2606-12. [PubMed]
- Lagerwaard FJ, Voet PW, van Meerbeeck JP, et al. Curative radiotherapy for a second primary lung cancer arising after pneumonectomy -- techniques and results. Radiother Oncol 2002;62:21-5. [PubMed]
- Haasbeek CJ, Lagerwaard FJ, de Jaeger K, et al. Outcomes of stereotactic radiotherapy for a new clinical stage I lung cancer arising postpneumonectomy. Cancer 2009;115:587-94. [PubMed]
- Senthi S, Haasbeek CJ, Lagerwaard FJ, et al. Radiotherapy for a second primary lung cancer arising post-pneumonectomy: planning considerations and clinical outcomes. J Thorac Dis 2013;5:116-22. [PubMed]
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833-9. [PubMed]
- Yano T, Okamoto T, Fukuyama S, et al. Therapeutic strategy for postoperative recurrence in patients with non-small cell lung cancer. World J Clin Oncol 2014;5:1048-54. [PubMed]
- Jiménez MF, Varela G, Novoa NM, et al. Results of surgery for non-small cell lung cancer with N2 involvement unsuspected before thoracotomy. Arch Bronconeumol 2008;44:65-9. [PubMed]
- Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010;28:2181-90. [PubMed]
- Griffioen GH, Toguri D, Dahele M, et al. Radical treatment of synchronous oligometastatic non-small cell lung carcinoma (NSCLC): patient outcomes and prognostic factors. Lung Cancer 2013;82:95-102. [PubMed]
- Kruser TJ, McCabe BP, Mehta MP, et al. Reirradiation for locoregionally recurrent lung cancer: outcomes in small cell and non-small cell lung carcinoma. Am J Clin Oncol 2014;37:70-6. [PubMed]
- McAvoy SA, Ciura KT, Rineer JM, et al. Feasibility of proton beam therapy for reirradiation of locoregionally recurrent non-small cell lung cancer. Radiother Oncol 2013;109:38-44. [PubMed]
- Mauguen A, Le Péchoux C, Saunders MI, et al. Hyperfractionated or accelerated radiotherapy in lung cancer: an individual patient data meta-analysis. J Clin Oncol 2012;30:2788-97. [PubMed]
- Jiang J, Liang X, Zhou X, et al. Non-platinum doublets were as effective as platinum-based doublets for chemotherapy-naïve advanced non-small-cell lung cancer in the era of third-generation agents. J Cancer Res Clin Oncol 2013;139:25-38. [PubMed]
- Yano T, Haro A, Yoshida T, et al. Prognostic impact of local treatment against postoperative oligometastases in non-small cell lung cancer. J Surg Oncol 2010;102:852-5. [PubMed]
- Ashworth AB, Senan S, Palma DA, et al. An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancer. Clin Lung Cancer 2014;15:346-55. [PubMed]
- Parikh RB, Cronin AM, Kozono DE, et al. Definitive primary therapy in patients presenting with oligometastatic non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2014;89:880-7. [PubMed]