PET-CT guided curative conformal radiation therapy in limited stage small cell lung cancer
Introduction
Lung cancer is the leading cause of cancer-related mortality. Although surgery, if available, is the cornerstone of the management of non-small cell lung cancer (NSCLC), the principal treatment procedures for small cell lung cancer (SCLC) are chemotherapy and radiation therapy (RT) (1,2).
The prognosis of SCLC is poor, with a reported median survival period of 2 to 4 months for untreated patients (3,4). However, the prognosis of limited disease small cell lung cancer (LD-SCLC) has improved significantly in recent decades, due to diagnostic and therapeutic advances (5,6). Better integration of chemotherapy and radiotherapy, such as delivering accelerated concurrent chemotherapy with chest irradiation and prophylactic whole-brain irradiation increases survival rates (7).
RT for SCLC consists of curative thoracic RT and prophylactic whole-brain RT. Thoracic RT includes three-dimensional (3D) conformal delineation of the tumor volume based on a 3D computed tomography (CT) image, and in a few patients, intensity modulated RT. Whatever the radiation delivery technique, the best diagnostic tool for accurate delineation of the tumor is the cornerstone of the RT (8,9).
Positron emission tomography (PET) imaging using 18F fluoro-2-deoxyglucose (18F-FDG) is a novel imaging technique that has been used in clinical oncology since the 1990s (10). In addition, the introduction of FDG-PET/CT with co-registration of PET and CT data has resulted in higher sensitivity and specificity. The impact of this imaging modality on cancer diagnosis, staging, and management has been substantial, and it has represented an important diagnostic modality worldwide (11). The combination of PET and CT allows both biological and anatomical confirmation of tumors on the same imaging tool, thereby defining the tumor and lymphatic and distant metastasis of the tumor more precisely. Moreover, in treatment management of cancer, especially in RT, FDG-PET/CT provides the most accurate target delineation (12). With accurate delineation of the tumor, FDG-PET/CT assists radiation oncologists in escalating the dose to the tumor, while sparing normal tissue.
The first important field in which FDG-PET/CT was found to be more sensitive and specific was in the diagnosis of lung cancer. The introduction of the term ‘‘biological target volume’’, which means target volume delineation based on metabolic information, expanded the application of FDG-PET/CT to many other cancers (13,14).
Curative thoracic chemoradiotherapy (CRT) is the standard of care for limited stage SCLC. However, local failure still occurs in 30-50% of patients, and toxicity of the therapy is a major concern (15). The main approach to improving local control and survival is dose escalation based on 3D conformal RT planning (16,17), while not increasing toxicity. At this point, the challenge in using this intervention is protecting the normal tissue from the escalated dose, which can be accomplished by defining the gross tumor volume (GTV) more accurately and precisely (18).
While FDG-PET/CT has been studied in NSCLC for a long time, it has only recently been applied to SCLC. A few studies have shown the impact of FDG-PET/CT in SCLC, especially in terms of radiation treatment planning and the prognostic significance of this imaging modality (10,18).
As such, we conducted a retrospective analysis to observe firstly the prognostic importance of the FDG uptake in LD-SCLC patients and share the clinical outcomes and toxicity profiles of these patients treated with curative thoracic RT using FDG-PET/CT simulation.
Patients and methods
The clinical records of all consecutive patients with SCLC who underwent external beam RT initiated at the Ataturk Chest Disease Research and Training Hospital between 2009 and 2011 were retrospectively reviewed. Complete staging procedures included history taking and physical examination, chest radiography, bone scintigraphy (if needed), magnetic resonance imaging (MRI) of the brain, and hematological and biochemical tests. The staging evaluation of all the patients indicated limited stage. None of the patients had a second primary cancer, nor had they been treated previously with chemotherapy and/or RT for a diagnosis of lung cancer.
Limited-stage small-cell lung cancer (LS-SCLC) was defined as disease confined to the thorax and regional nodes, without malignant pleural effusion. The patients were included in the analysis if they were diagnosed with histologically proven LS-SCLC that had not been treated previously with RT, had undergone staging using PET/CT before RT, had undergone definitive 3D conformal RT for the primary disease, and had complete RT records available.
The patients underwent 3D-CRT using the Xio-CT-based treatment planning software program (Stockholm, Sweden). They underwent PET/CT, simulation and treatment in the supine position, with their arms raised above their heads, and they were immobilized using a wing board (Medtec, Orange City, IA, USA).
The GTV included regions of primary disease and nodal metastasis defined by metabolically active regions on the patient’s staging PET/CT scans. In some patients, involved nodal regions diagnosed by histological evaluation of the biopsy samples obtained during mediastinoscopy or bronchoscopy were included in the GTV; nodes showing 18F-fluorodeoxyglucose avidity on the PET/CT scans were also included in the GTV.
Typically, the clinical target volume (CTV) was defined as the internal GTV plus an 8-mm margin. The CTV was then expanded by 0.5-1.0 cm, to compensate for setup variability and target motion, to generate the planning target volume (PTV). The plans were corrected for tissue inhomogeneity during treatment planning.
Radiation was delivered using 6-18-MV photon beams from a linear accelerator. Thoracic radiation was administered at a daily fraction of 2 Gy. Total dose was prescribed according to the chemotherapy protocol whether it is concurrent or sequential. Some patients referred for RT after full dose chemotherapy (4-6 cycles) with a total or near total response after chemotherapy. And these patients received lower thoracic doses. Other patients, who had no response or minimal response, received 60 Gy when concurrent chemotherapy was administered or higher than 60 Gy when sequential chemotherapy was applied. Only a few patients in this group received 54-58 Gy due to the limitations of OAR doses. During RT, we evaluated the patients weekly for acute toxic effects. After the end of the chemotherapy-radiotherapy, the patients were evaluated for response. If there was no disease progression and response to the treatment was observed, the patients generally were offered prophylactic cranial RT at a total dose of 25 Gy in 2.5 Gy/10 fractions.
All patients underwent induction chemotherapy, concurrent CRT or sequential CRT which consisted of six cycles of chemotherapy and thoracic RT. Most of the patients referred for RT after 4-6 cycles of chemotherapy and they underwent sequential CRT. And patients with co-morbidities and older ages underwent sequential CRT.
Patients underwent combined chemotherapy with a cisplatin and etoposide (EP) regimen as the first-line treatment. The EP regimen consisted of EP 100 mg/m2 on days 1 to 3, and cisplatin 70 mg/m2 on day 1. Both regimens were repeated every 3 weeks.
After completion of the treatment, the patients were evaluated with repeat clinical examinations and imaging every 3 months for 2 years, and then every 6 months for 3 years. Tumor recurrences and systemic metastases were recorded, and patients underwent chemotherapy and palliative radiotherapy. Acute, subacute, and late toxicity were evaluated, and if evident, scored according to the Radiation Therapy Oncology Group (RTOG) Acute Radiation Morbidity and the European Organization for Research and Treatment of Cancer (EORTC) & RTOG Late Radiation Morbidity (19).
The clinical endpoints were overall survival (OS), relapse-free survival (RFS), and disease-free survival (DFS). Recurrence or progression in the thorax was classified as relapse, and both relapse and metastasis were classified as disease. Survival and time to failure were measured from the date of the pathological diagnosis of SCLC, and disease (relapse and metastasis) timing was defined as the time of occurrence of the disease on first imaging or clinical finding. Survival time was estimated using the Kaplan-Meier estimator (20). Survival differences among the subgroups of patients were assessed using the log-rank test (21). Multivariate analysis of survival was calculated using the Cox proportional hazards model (22). Statistical analyses were performed with the SPSS for Windows software program version 15 (Chicago, USA).
Results
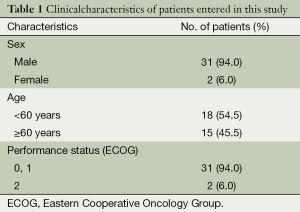
A total of 33 patients with SCLC, treated using 3D-CRT guided by PET/CT between 2009 and 2011, were included in the present study. Patient characteristics are listed in Table 1. None of the patients died early due to treatment-related toxicities or refused to continue the treatment; therefore, all of the patients were on follow-up and analyzed.
Full table
The median age of the patients was 58 years (range, 38-77 years). Most patients had a good performance status, with 94% of them having an Eastern Cooperative Oncology Group score of 0 or 1. Tumor localization was on the left lung in 19 (57.6%) of the patients, and on the right lung in the remaining ones.
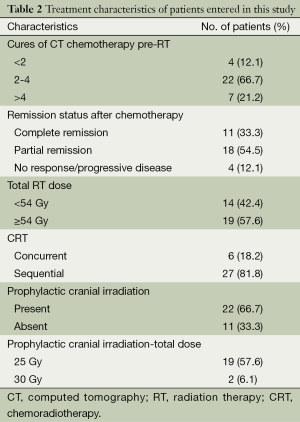
Most of the patients (66.7%) had nodal disease at treatment, mediastinal and/or hilar. All of the patients were staged as LS-SCLC after the initial workup. All of the patients received induction CT; the median cure number was 4 (range, 1-6) cures. Six patients also underwent concurrent chemotherapy; all of the concurrent chemotherapy regimens were cisplatin and EP. The induction chemotherapy regimen was also cisplatin and EP.
All of the patients received thoracic radiation, 2Gy daily fractionated, with a median total prescribed radiation dose of 54 Gy (range, 46-70 Gy). Of the 33 patients, who showed complete or partial remission after CRT, it was decided that 23 should have prophylactic cranial irradiation (PCI). However, PCI could not be planned for a patient who had cerebrovascular disease, so 22 patients received it; 21 patients (63.7%) received the full dose. The most frequently prescribed PCI radiation dose was 2.5 Gy per fraction, for a total dose of 25 Gy. Two patients received a 30-Gy total dose, in 2-Gy daily fractions. Details of the chemotherapy regimens and RT schedules are summarized in Table 2.
Full table
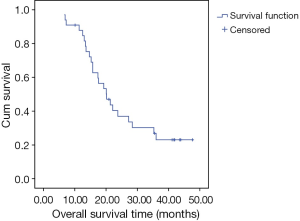
The median follow up time was 20 months (range, 6.6-47.6 months). The median survival period was 20.16 months [95% confidence interval (CI), 15.06-25.27] (Figure 1). The 3-year OS and locoregional control rates were 23% and 48%, respectively.
The median size of the primary tumor on the initial diagnosis was 5 cm (range, 3-10 cm). The response of the tumor to induction chemotherapy was more than 50% in 17 patients (51.5%), and complete remission was seen in 11 patients (33.3%). The median size of the primary tumor before RT planning was 3 cm (range, 1-9.5 cm). The median primary tumor standardized uptake value (SUVmax) of the tumor was 5.9 (range, 1.22-19.06). The median SUVmax values of the mediastinal and hilar lymph nodes were 5 (range, 2.25-9.32) and 3.2 (range, 2.48-8.56), respectively.
Toxicity of the thoracic RT manifested as grade I esophagitis in 16 patients (48.5%). One patient (3%) experienced grade II dermatitis, and grade II esophagitis appeared in one patient (3%). There was no grade III or grade IV toxicity due to the thoracic irradiation or PCI. There was no treatment-related death due to the thoracic RT or PCI.
Local recurrence or progression was seen in 11 patients (33.3%), and 15 patients (45.5%) experienced metastatic disease. The leading cause of treatment failure was metastatic disease, which involved nearly half of the patients. Brain metastasis was observed in four patients, and liver metastasis was observed in four patients.
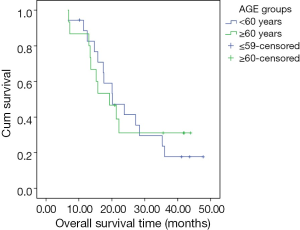
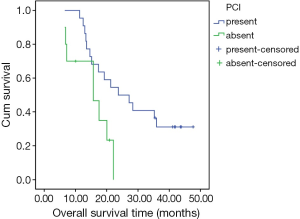
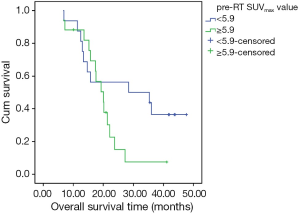
A univariate analysis of OS determined that age and gender were not significant predictors of survival. There was no significant difference in survival between groups defined according to age (<60 and ≥60 years, P=0.921) (Figure 2). Treatment response to induction chemotherapy also was not a significant predictor of survival. However, PCI was found to be a significant predictor of median survival. Median survival was 23.8 months (95% CI, 13.3-34.3) in the group that received PCI, versus the shorter median survival period of 15.7 months (95% CI, 6.1-25.3) in the group without PCI (P=0.025) (Figure 3). The median SUVmax value of the tumor (5.9) was decided as a cutoff value, and the impact of the SUVmax value on survival was statistically evaluated. There was no statistically significant difference in survival between the low (<5.9) and high (≥5.9) SUVmax groups (P=0.104). On the other hand, although not statistically significant, the 2-year OS rate was lower in the high SUVmax group (56% and 15% in the low and high SUVmax groups, respectively) (Figure 4).
Discussion
In this study, we retrospectively examined the potential impact of FDG-PET/CT on the treatment outcomes of treatment response and toxicity in a group of patients with LS-SCLC who were undergoing curative CRT.
Our SCLC patient data showed a 3-year OS rate of 23%. Our results are comparable with other studies of LD-SCLC patients (23,24) published in the literature. There is also a wide range of survival rates reported in the literature, due to different treatment strategies, therapeutic regimens, and selection of patients among oncology centers. The 3- and 4-year locoregional control rates in our study were 48% and 27%, respectively. Except for one study, which reported a 5-year locoregional control rate of 52%, studies including LD-SCLC have reported lower locoregional control rates, despite twice or daily RT fractionation schemas (25-27). The maximum total dose applied with 1.8 Gy once a day was 50.4 Gy in the studies. When compared with the literature, our locoregional control rates were higher. Our explanation for these differences and for better locoregional control is the precise delineation of the tumor and lymphatics with PET-CT simulation, as well as the higher thoracic doses prescribed, especially in patients with high tumor SUVmax values.
PCI was applied to 22 patients (63.7%); brain metastasis developed in two of these patients (9.1%). On the other hand, there were 3 (27.3%) cases of brain metastasis detected in the remaining 11 patients, who did not receive PCI. The median survival rate of the PCI patients was better than that of the group without PCI (23.8 vs. 15.7 months, respectively); the difference was statistically significant. Our results are in agreement with the literature data, which suggests that PCI is an important prognostic indicator of survival in both LD-SCLC and extended disease-small cell lung cancer (ED-SCLC) patients (28,29). For patients who respond to CT, whether completely or partially, PCI must be included in the treatment schedule.
We could not demonstrate a statistically significant poorer survival related to high SUVmax value. However, the 2-year OS was better in the low SUVmax group than in the high SUVmax group (56% vs. 15%, respectively). This subject was evaluated in a similar retrospective study of 41 LD-SCLC and 35 ED-SCLC patients with combined CRT and PCI. In that study, the pre-RT tumor SUVmax value was found to be statistically significant. The median SUVmax value of the tumor (8.7) was accepted as the cutoff value for identifying the high and low SUVmax groups, and statistical analysis showed that the high SUVmax group had poorer survival outcomes (10). In our study, although the survival rate was lower in the high SUVmax group, a statistically significant difference could not be shown. This result might be due to the high total thoracic RT doses in our study, which are slightly higher than the doses reported in other studies in the literature (27). Most of the patients in our high SUVmax group received ≥54 Gy daily fractions of 2 Gy (median 60 Gy; range, 54-70 Gy) in high SUVmax group.
Even in limited disease cases, the prognosis for SCLC is poor. However, with the improving effectiveness of chemotherapeutic agents, advances in RT, and with better definition of the disease due to advances in imaging technology, long-term survival has been improving. Combining chemotherapy with early thoracic RT and PCI is the accepted and recommended therapeutic application in LD-SCLC. In addition, in ED-SCLC, if the treatment response is complete, thoracic RT and PCI are offered (3).
Only six patients received chemotherapy and thoracic RT concurrently in our study. However, the survival rates are comparable to the rates reported in previous studies. To improve the outcome of LD-SCLC patients, more intensive therapy concepts must be evaluated. One of these concepts, multidrug CT concurrently with thoracic RT, was reported by Turrisi et al., who observed a survival advantage with early hyperfractionated chest CRT (7). However, toxicity may be a challenging issue, and conventionally fractionated RT is also suitable for LD-SCLC patients. In this situation, higher doses are needed for locoregional control. Better local control rates have been demonstrated with a 60 Gy thoracic dose (30) and 70 Gy has also been shown to be safe in these patients. However, higher doses are related to higher toxicities. As such, a more accurate delineation of the tumor and positive lymph nodes is a major concern in RT planning. Precisely targeting the tumor will allow higher doses without increasing toxicity. In this manner, more advanced imaging technology helps clinicians. The introduction of PET and PET/CT hybrid scanners for metabolic tumor imaging has facilitated more precise tumor contouring for radiation oncologists. Another important advantage of PET/CT is the ability to escalate doses to the levels necessary to contain active tumor cells without increasing the toxicity of neighboring organs. Clinical trials on lung cancer, especially NSCLC, have demonstrated that patients who receive PET-based radiation treatment have better OS rates (31). PET-assisted staging also assists in the management of patients and impacts radical RT planning (32). In addition to lung cancer, the cost-effectiveness of the clinical use of PET has been demonstrated in other malignancies, such as lymphoma and colorectal carcinoma (10,12).
In NSCLC, 18F-PET/CT is effectively used for staging, RT planning, and restaging, due to its high sensitivity and specificity when compared to conventional imaging techniques (1). More recently, with the important gains of this functional imaging modality that have been made with NSCLC, it has been increasingly applied in SCLC for management of the disease and FDG uptake, showing that PET can be used as a prognostic indicator of patient survival (10,33-35). According to this finding, higher RT doses in response to higher FDG uptake might also have a major impact on locoregional control.
Due to the retrospective nature of our study, there are several important limitations. The first is that nodal pathological confirmation was conducted in only two patients, except for 16 patients with nodal FDG uptake. Secondly, most of the study population could not receive early thoracic RT, due to the larger tumor sizes and lack of coordination between departments. Although all of the patients were treated in the same RT department, the RT doses were different, due to limiting organ toxicity in some patients and the different management programs of various radiation oncologists. The last limitation is the limited number of patients and limited follow-up. LS-SCLC is a relatively rare condition in the general population and in the lung cancer population, and poor survival of SCLC patients limits long-term follow-up of these patients.
Although SCLC is not higher than 20% of all lung cancers, the rapid growth and poor survival rate makes the disease noteworthy. Intensified therapies to improve both locoregional control and systemic control are under research. Results of our study and of the literature show that the major cause of treatment failure after combined CRT and PCI is metastatic spread. Essentially, observations of systemic treatment appear to be the main problem, and secondly, locoregional therapy must be evaluated in larger, prospective, randomized studies.
Conclusions
There are few studies examining the impact of PET-CT and the prognostic significance of FDG-uptake on outcomes in patients with LD-SCLC. Higher RT doses in response to higher FDG uptake may be a safe approach for the purpose of locoregional control.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Kerhet A, Small C, Quon H, et al. Application of machine learning methodology for PET-based definition of lung cancer. Curr Oncol 2010;17:41-7. [PubMed]
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. [PubMed]
- van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet 2011;378:1741-55. [PubMed]
- Kato Y, Ferguson TB, Bennett DE, et al. Oat cell carcinoma of the lung. A review of 138 cases. Cancer 1969;23:517-24. [PubMed]
- De Ruysscher D, Vansteenkiste J. Chest radiotherapy in limited-stage small cell lung cancer: facts, questions, prospects. Radiother Oncol 2000;55:1-9. [PubMed]
- Erridge SC, Murray N. Thoracic radiotherapy for limited-stage small cell lung cancer: issues of timing, volumes, dose, and fractionation. Semin Oncol 2003;30:26-37. [PubMed]
- Turrisi AT 3rd, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 1999;340:265-71. [PubMed]
- van Baardwijk A, Baumert BG, Bosmans G, et al. The current status of FDG-PET in tumour volume definition in radiotherapy treatment planning. Cancer Treat Rev 2006;32:245-60. [PubMed]
- Nestle U, Kremp S, Grosu AL. Practical integration of [18F]-FDG-PET and PET-CT in the planning of radiotherapy for non-small cell lung cancer (NSCLC): the technical basis, ICRU-target volumes, problems, perspectives. Radiother Oncol 2006;81:209-25. [PubMed]
- Lee YJ, Cho A, Cho BC, et al. High tumor metabolic activity as measured by fluorodeoxyglucose positron emission tomography is associated with poor prognosis in limited and extensive stage small-cell lung cancer. Clin Cancer Res 2009;15:2426-32. [PubMed]
- von Schulthess GK, Steinert HC, Hany TF. Integrated PET/CT: current applications and future directions. Radiology 2006;238:405-22. [PubMed]
- Buck AK, Herrmann K, Stargardt T, et al. Economic evaluation of PET and PET/CT in oncology: evidence and methodologic approaches. J Nucl Med 2010;51:401-12. [PubMed]
- Rege SD, Hoh CK, Glaspy JA, et al. Imaging of pulmonary mass lesions with whole-body positron emission tomography and fluorodeoxyglucose. Cancer 1993;72:82-90. [PubMed]
- Nolop KB, Rhodes CG, Brudin LH, et al. Glucose utilization in vivo by human pulmonary neoplasms. Cancer 1987;60:2682-9. [PubMed]
- De Ruysscher D, Bremer RH, Koppe F, et al. Omission of elective node irradiation on basis of CT-scans in patients with limited disease small cell lung cancer: a phase II trial. Radiother Oncol 2006;80:307-12. [PubMed]
- Rodríguez N, Sanz X, Trampal C, et al. 18F-FDG PET definition of gross tumor volume for radiotherapy of lung cancer: is the tumor uptake value-based approach appropriate for lymph node delineation? Int J Radiat Oncol Biol Phys 2010;78:659-66. [PubMed]
- Socinski MA, Morris DE, Halle JS, et al. Induction and concurrent chemotherapy with high-dose thoracic conformal radiation therapy in unresectable stage IIIA and IIIB non-small-cell lung cancer: a dose-escalation phase I trial. J Clin Oncol 2004;22:4341-50. [PubMed]
- Socinski MA, Blackstock AW, Bogart JA, et al. Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non-small-cell lung cancer: CALGB 30105. J Clin Oncol 2008;26:2457-63. [PubMed]
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys 1995;31:1341-6. [PubMed]
- Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 1966;50:163-70. [PubMed]
- Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. J Am Stat Assoc 1958;53:457-81.
- Cox DR. Regression Models and Life-Tables. J R Stat Soc Series B Stat Methodol 1972;34:187-220.
- Miller KL, Marks LB, Sibley GS, et al. Routine use of approximately 60 Gy once-daily thoracic irradiation for patients with limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys 2003;56:355-9. [PubMed]
- Spiro SG, James LE, Rudd RM, et al. Early compared with late radiotherapy in combined modality treatment for limited disease small-cell lung cancer: a London Lung Cancer Group multicenter randomized clinical trial and meta-analysis. J Clin Oncol 2006;24:3823-30. [PubMed]
- Arriagada R, Pellae-Cosset B, Ladron de Guevara JC, et al. Alternating radiotherapy and chemotherapy schedules in limited small cell lung cancer: analysis of local chest recurrences. Radiother Oncol 1991;20:91-8. [PubMed]
- Bonner JA, Sloan JA, Shanahan TG, et al. Phase III comparison of twice-daily split-course irradiation versus once-daily irradiation for patients with limited stage small-cell lung carcinoma. J Clin Oncol 1999;17:2681-91. [PubMed]
- Herrmann MK, Bloch E, Overbeck T, et al. Mediastinal radiotherapy after multidrug chemotherapy and prophylactic cranial irradiation in patients with SCLC--treatment results after long-term follow-up and literature overview. Cancer Radiother 2011;15:81-8. [PubMed]
- Aupérin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999;341:476-84. [PubMed]
- Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med 2007;357:664-72. [PubMed]
- Papac RJ, Son Y, Bien R, et al. Improved local control of thoracic disease in small cell lung cancer with higher dose thoracic irradiation and cyclic chemotherapy. Int J Radiat Oncol Biol Phys 1987;13:993-8. [PubMed]
- Mac Manus MP, Wong K, Hicks RJ, et al. Early mortality after radical radiotherapy for non-small-cell lung cancer: comparison of PET-staged and conventionally staged cohorts treated at a large tertiary referral center. Int J Radiat Oncol Biol Phys 2002;52:351-61. [PubMed]
- MacManus M, Nestle U, Rosenzweig KE, et al. Use of PET and PET/CT for radiation therapy planning: IAEA expert report 2006-2007. Radiother Oncol 2009;91:85-94. [PubMed]
- Bradley JD, Dehdashti F, Mintun MA, et al. Positron emission tomography in limited-stage small-cell lung cancer: a prospective study. J Clin Oncol 2004;22:3248-54. [PubMed]
- Ung YC, Maziak DE, Vanderveen JA, et al. 18Fluorodeoxyglucose positron emission tomography in the diagnosis and staging of lung cancer: a systematic review. J Natl Cancer Inst 2007;99:1753-67. [PubMed]
- Ulger S, Demirci NY, Eroglu FN, et al. High FDG uptake predicts poorer survival in locally advanced nonsmall cell lung cancer patients undergoing curative radiotherapy, independently of tumor size. J Cancer Res Clin Oncol 2014;140:495-502. [PubMed]
PET-CT guided curative conformal radiation therapy in limited stage small cell lung cancer. J Thorac Dis 2015;7(3):295-302. doi: 10.3978/j.issn.2072-1439.2015.02.02